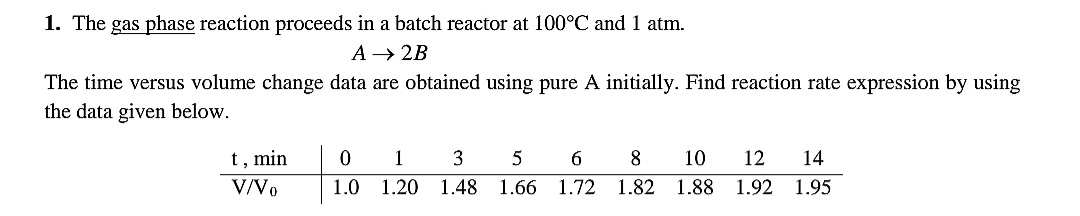
Question: The gas phase reaction proceeds in a batch reactor at 1 0 0 C and 1 atm. A 2 B The time versus volume change
The gas phase reaction proceeds in a batch reactor at and atm.
The time versus volume change data are obtained using pure A initially. Find reaction rate expression by using the data given below.
tablemin,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


