Question: The graph below was obtained in an experiment studying the reaction NH1NCO(aq)(NH2)2CO(aq) at a particular temperature NCO, and the rate constant is The graph below
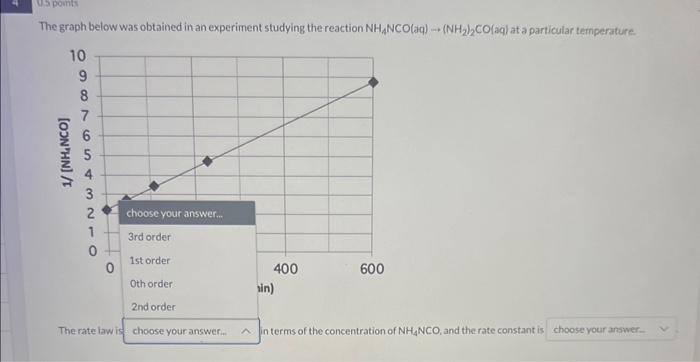
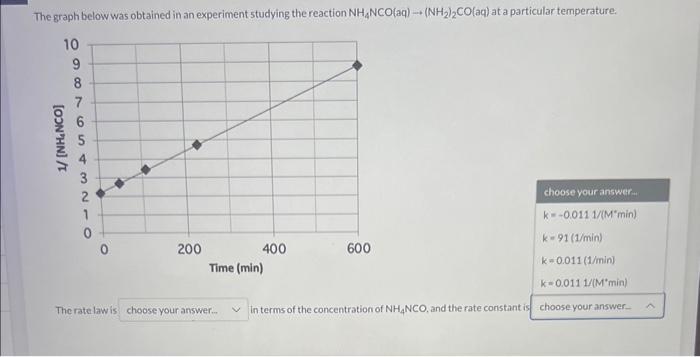
The graph below was obtained in an experiment studying the reaction NH1NCO(aq)(NH2)2CO(aq) at a particular temperature NCO, and the rate constant is The graph below was obtained in an experiment studying the reaction NH4NCO(aq)(NH2)2CO(aq) at a particular temperature. choose your answer.. k=0.0111/(Mmin)k=91(1/min)k=0.011(1/min)k=0.0111(Mmin) The rate law is in terms of the concentration of NH4NCO, and the rate constant is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


