Question: The heat flow, through a layer of material, by conduction is given by: = A (TH-TC) R Where 'Q' is the heat, 't' is
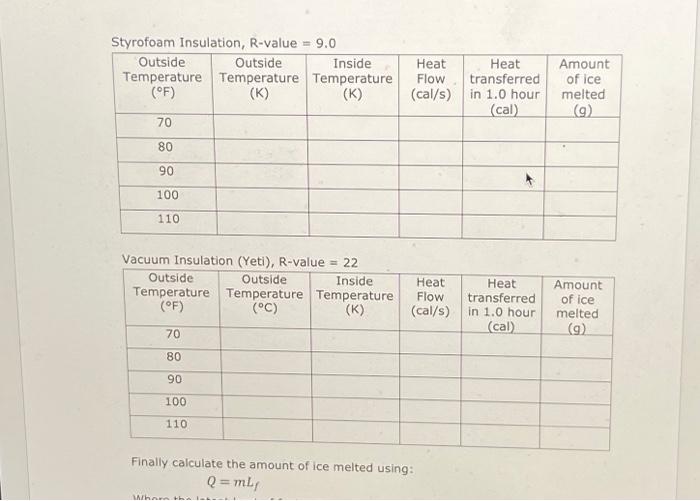
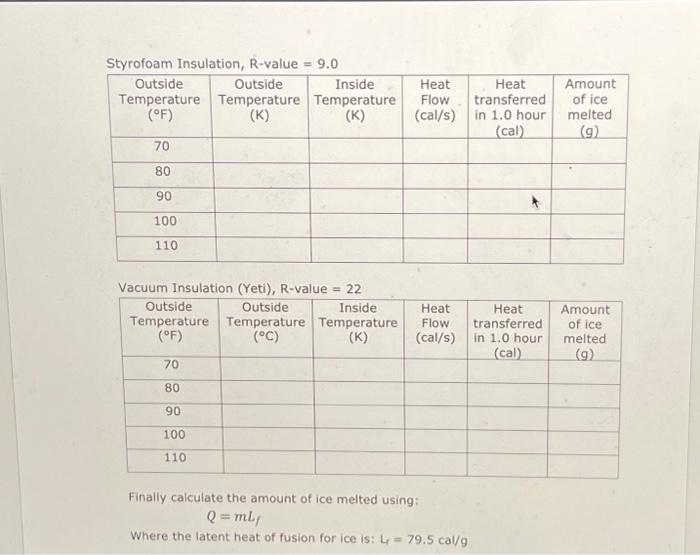

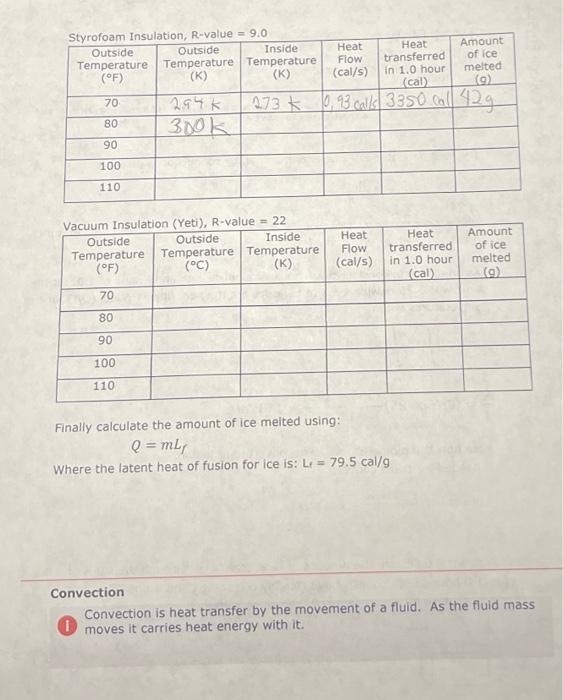

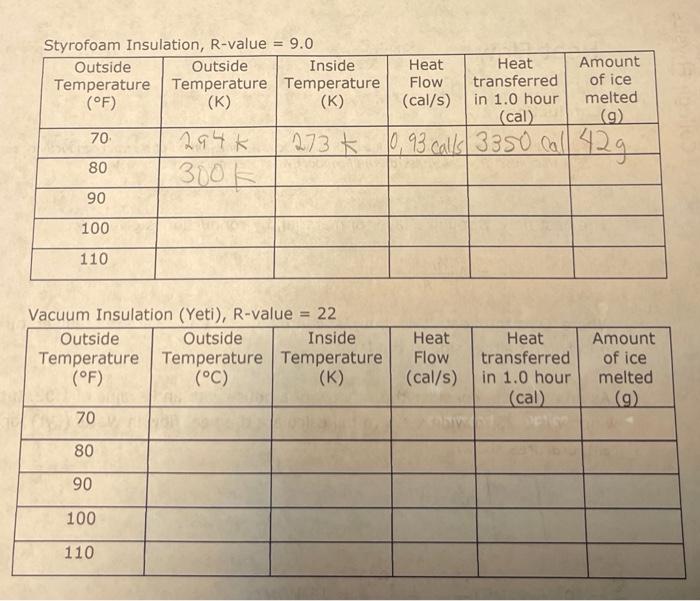
The heat flow, through a layer of material, by conduction is given by: = A (TH-TC) R Where 'Q' is the heat, 't' is time, 'A' is area and TH and Te are the temperatures in Kelvin on either side of the layer. As an example, consider the window of a house with an R-value of 1.02. If the window is 0.75 m wide x 1.5 m tall calculate the heat flow in Watts (Q/t) for the temperatures shown. TH 35 C 0 =(0.75 (0.75 x 1.5) Tc=25 C 11watts= (306-296) 1.02 Note how the temperatures were converted to Kelvin. If needed we could convert = 11 Watts 114x 0.239 Cal 1/ = 2.6 Cal/s. Styrofoam Insulation, R-value = 9.0 Outside Outside Inside Temperature Temperature Temperature (F) (K) (K) 70, 80 90 100 110 70 80 294 K 300K Vacuum Insulation (Yeti), R-value = 22 Outside Outside Inside Temperature Temperature Temperature (F) (C) (K) 90 100 110 273 Amount of ice melted (cal) (g) 0,93 calls 3350 call 42g Heat Flow (cal/s) Heat Flow (cal/s) Heat transferred in 1.0 hour Heat transferred in 1.0 hour (cal) Amount of ice melted (g) Styrofoam Insulation, R-value = 9.0 Outside Outside Inside Temperature Temperature Temperature (F) (K) 70 80 90 100 110 70 80 90 100 110 294k 300k Vacuum Insulation (Yeti), R-value = 22 Outside Outside Inside Temperature Temperature Temperature (F) (C) (K) Convection Heat Flow (cal/s) Heat transferred melted in 1.0 hour (cal) (9) 2730,93 call 3350 call 42g Heat Flow (cal/s) Heat transferred in 1.0 hour (cal) Finally calculate the amount of ice melted using: Q = mL, Where the latent heat of fusion for ice is: L = 79.5 cal/g Amount of ice Amount of ice melted (9) Convection is heat transfer by the movement of a fluid. As the fluid mass moves it carries heat energy with it. Conduction 0 Thermal Resistance or R-value. The R-value is a combination of thermal conductivity and thickness. R = thickness/thermal conductivity. Metric units are mK/W The heat flow, through a layer of material, by conduction is given by: = A (TH-TC) R Where 'Q' is the heat, 't' is time, 'A' is area and TH and Tc are the temperatures in Kelvin on either side of the layer. As an example, consider the window of a house with an R-value of 1.02. If the window is 0.75 m wide x 1.5 m tall calculate the heat flow in Watts (Q/t) for the temperatures shown. 0 = (0.75 1.5) TH=35 C Tc=25 C 11watts = (306-296) 1.02 Note how the temperatures were converted to Kelvin. If needed we could convert = 11 Watts 0.239 Cal = 2.6 Cal/s. Styrofoam Insulation, R-value = 9.0 Outside Outside Inside Temperature Temperature Temperature (F) (K) (K) 70 80 90 100 110 Vacuum Insulation (Yeti), R-value = 22 Outside Outside Inside Temperature Temperature Temperature (F) (C) (K) 70 80 90 100 110 Heat Flow (cal/s) Heat Flow (cal/s) Finally calculate the amount of ice melted using: Q = mL, Where the latent heat of fusion for ice is: L 79.5 cal/g Heat transferred in 1.0 hour (cal) Heat transferred in 1.0 hour (cal) Amount of ice melted (9) Amount of ice melted (9) Styrofoam Insulation, R-value = 9.0 Outside Outside Inside Temperature Temperature Temperature (F) (K) (K) 70 80 90 100 110 Vacuum Insulation (Yeti), R-value = 22 Outside Outside Inside Temperature Temperature Temperature (F) (C) (K) 70 80 90 100 110 Heat Heat Flow transferred (cal/s) in 1.0 hour (cal) Whorn thi Heat Flow (cal/s) Finally calculate the amount of ice melted using: Q=mLf Heat transferred in 1.0 hour (cal) Amount of ice melted (9) Amount of ice melted (g)
Step by Step Solution
3.45 Rating (164 Votes )
There are 3 Steps involved in it
Using Styrofoam insulation and vacuum insulation Yeti we can quantify the amount of melted ice by fi... View full answer

Get step-by-step solutions from verified subject matter experts


