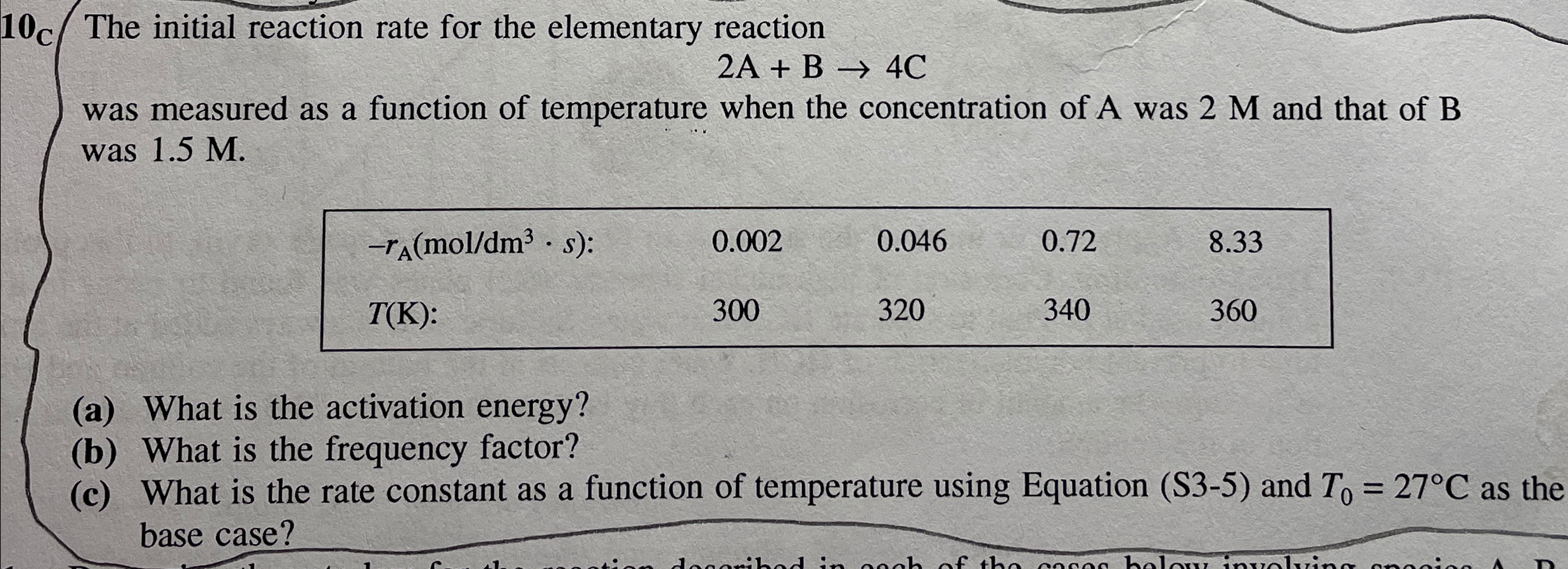
Question: The initial reaction rate for the elementary reaction 2 A + B 4 C was measured as a function of temperature when the concentration of
The initial reaction rate for the elementary reaction
was measured as a function of temperature when the concentration of A was and that of was
table::
a What is the activation energy?
b What is the frequency factor?
c What is the rate constant as a function of temperature using Equation S and as the base case?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


