Question: The ionic equation for the reaction between ( mathrm{LiOH} ) and Saicylaldehyde is given as: Molar mass of ( mathrm{LiOH} . mathrm{H}_{2} mathrm{O}=41.965 mathrm{~g} /
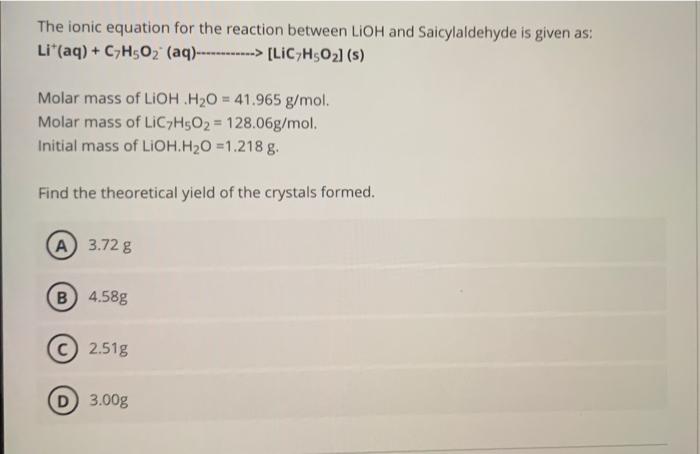
The ionic equation for the reaction between LiOH and Saicylaldehyde is given as: Li+(aq)+C7H5O2(aq)[LiC7H5O2] (s) Molar mass of LiOH.H2O=41.965g/mol. Molar mass of LiC7H5O2=128.06g/mol. Initial mass of LiOH.H2O=1.218g. Find the theoretical yield of the crystals formed. (A) 3.72g (B) 4.58g (C) 2.51g 3.00g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


