Question: *The iron(III) chloride solution is initially clear and yellow while the potassium thiocyanate solution is initially clear and colorless. (4pts) For the reaction of
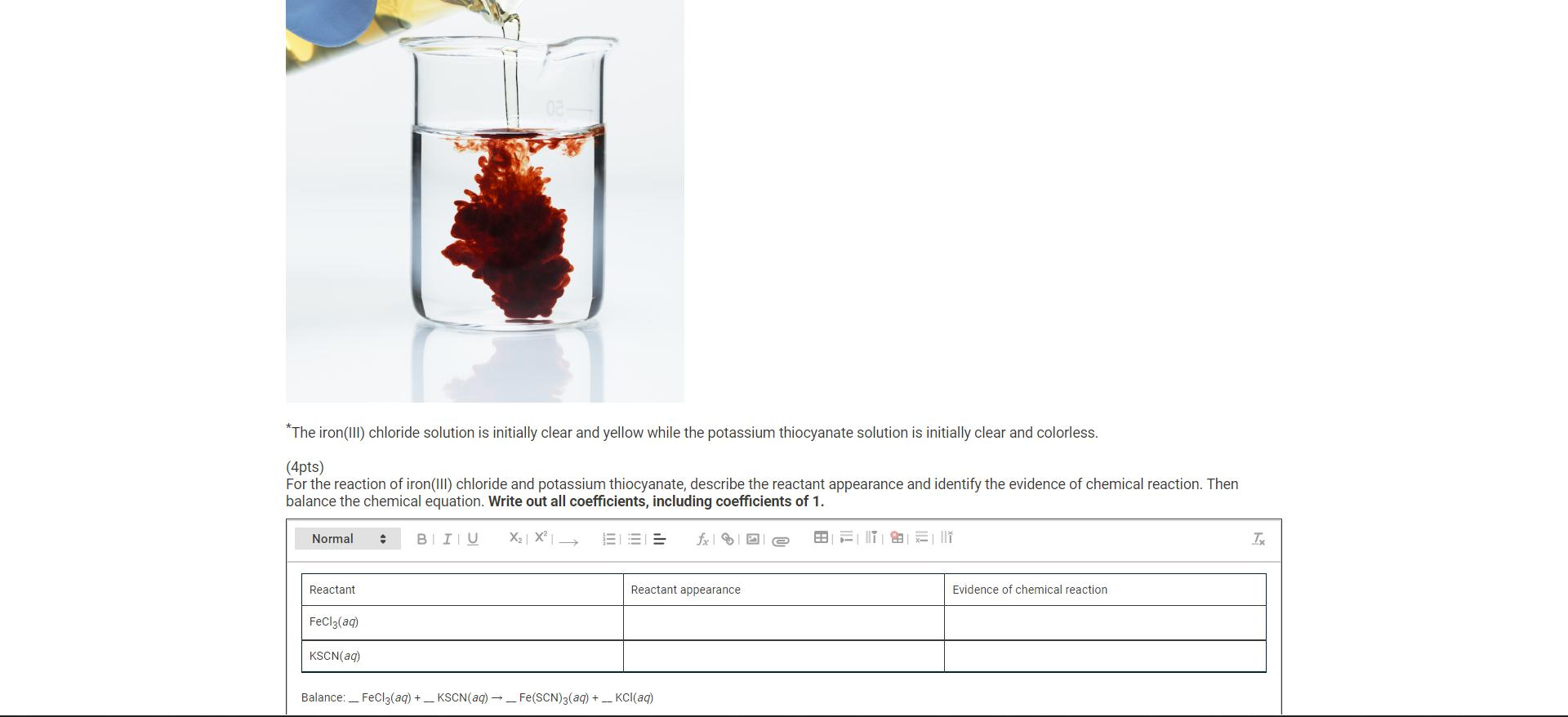
*The iron(III) chloride solution is initially clear and yellow while the potassium thiocyanate solution is initially clear and colorless. (4pts) For the reaction of iron(III) chloride and potassium thiocyanate, describe the reactant appearance and identify the evidence of chemical reaction. Then balance the chemical equation. Write out all coefficients, including coefficients of 1. Normal : BIU X X Reactant FeCl3(aq) KSCN(aq) Reactant appearance Balance: FeCl3(aq) +_ KSCN(aq) Fe(SCN)3(aq) + __ KCl(aq) Evidence of chemical reaction Tx
Step by Step Solution
3.39 Rating (152 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is ... View full answer

Get step-by-step solutions from verified subject matter experts


