Question: The kinetic parameters, k 1 , k 2 and k 3 at 1 0 0 C are given as 2 . 0 , 5 1
The kinetic parameters, and at are given as
and respectively. The
reactor feed is a slurry containing of and
of The concentration of is The reactor
conversion is Determine the reactor size and total
charge weight necessary to produce the product at an
and it will be necessary to shut down for between batches
for product removal, cleaning, and startup. The product R to
be recycled to the reactor will come from produced
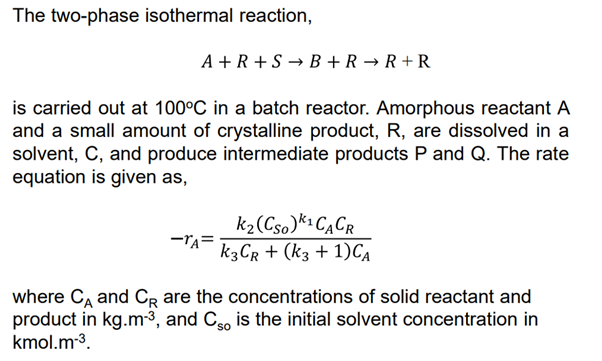
daily.The twophase isothermal reaction,
is carried out at in a batch reactor. Amorphous reactant
and a small amount of crystalline product, are dissolved in a
solvent, and produce intermediate products and The rate
equation is given as
where and are the concentrations of solid reactant and
product in and is the initial solvent concentration in
kmol.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


