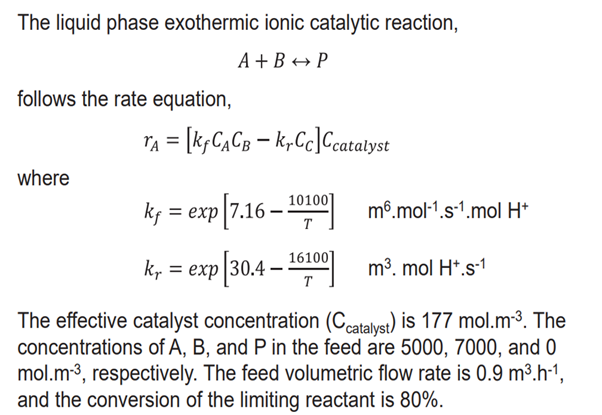
Question: The liquid phase exothermic ionic catalytic reaction, A + BharrP follows the rate equation, r A = [ k f C A C B -
The liquid phase exothermic ionic catalytic reaction,
BharrP
follows the rate equation,
where
exp
exp
The effective catalyst concentration is The
concentrations of and in the feed are and
mol. respectively. The feed volumetric flow rate is
and the conversion of the limiting reactant is The concentrations of and in the feed are
and mol. respectively.
The effective catalyst concentration of is mol.m
The feed volumetric flow rate is and the conversion of
the limiting reactant is
a Determine the CSTR volume at the temperature that
maximizes the reaction rate.
b Determine the PFR volume at the temperature that
maximizes the reaction rate.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


