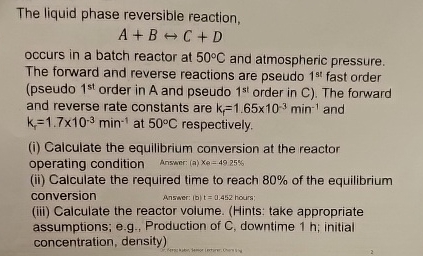
Question: The liquid phase reversible reaction, A + BharrC + D occurs in a batch reactor at 5 0 C and atmospheric pressure. The forward and
The liquid phase reversible reaction,
BharrC
occurs in a batch reactor at and atmospheric pressure. The forward and reverse reactions are pseudo fast order pseudo order in A and pseudo order in The forward and reverse rate constants are and at respectively.
i Calculate the equilibrium conversion at the reactor operating condition
Anssmer a
ii Calculate the required time to reach of the equilibrium conversion
Ansner: hours:
iii Calculate the reactor volume. Hints: take appropriate assumptions; eg Production of downtime ; initial concentration, density
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


