Question: (5) There is an elementary, reversible reaction A - B. At equilibrium, CA -0.1 moldim and Cer=0.5 mol/dm Which rate constant is larger? (5 points)
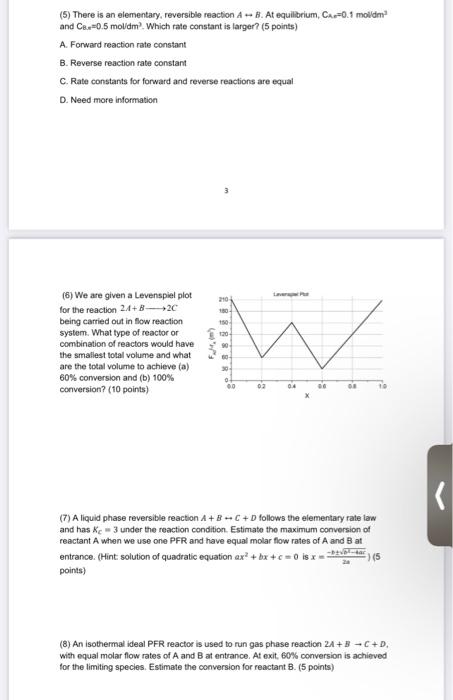
(5) There is an elementary, reversible reaction A - B. At equilibrium, CA -0.1 moldim and Cer=0.5 mol/dm Which rate constant is larger? (5 points) A. Forward reaction rate constant B. Reverse reaction rate constant C. Rate constants for forward and reverse reactions are equal D. Need more information 210 (8) We are given a Levenspiel plot for the reaction 24+20 being carried out in flow reaction system. What type of reactor or combination of reactors would have the smallest total volume and what are the total volume to achieve (a) 60% conversion and (b) 100% conversion? (10 points) 150 130 90 w M 30 00 02 (7) A liquid phase reversible reaction A + B - C + D follows the elementary rate law and has ke-3 under the reaction condition. Estimate the maximum conversion of reactant A when we use one PFR and have equal molar flow rates of A and B at entrance. (Hint solution of quadratic equation ax +bx+c 0ix-- points) (8) An isothermal ideal PFR reactor is used to run gas phase reaction 2A + B - C+D. with equal molar flow rates of A and B at entrance. At exit, 60% conversion is achieved for the limiting species. Estimate the conversion for reactant B. (5 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


