Question: The method of initial rates is a method that allows us to determine the rate law for a reaction based on a series of experimental
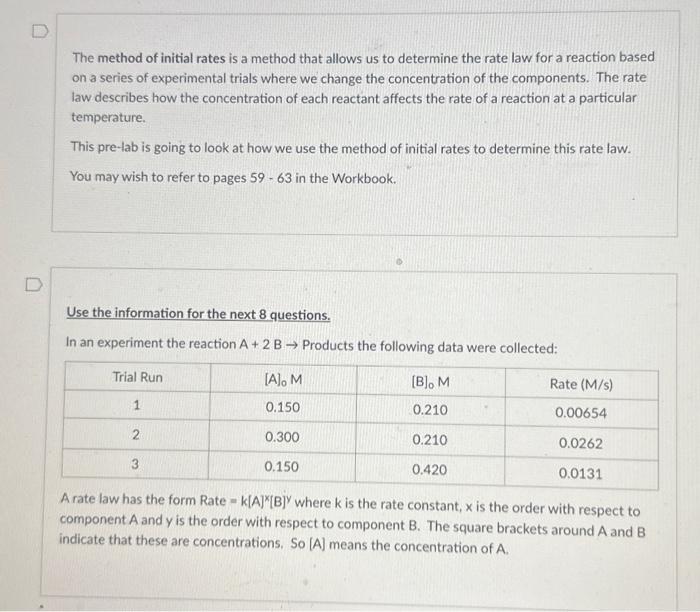

The method of initial rates is a method that allows us to determine the rate law for a reaction based on a series of experimental trials where we change the concentration of the components. The rate law describes how the concentration of each reactant affects the rate of a reaction at a particular temperature. This pre-lab is going to look at how we use the method of initial rates to determine this rate law. You may wish to refer to pages 59 - 63 in the Workbook. Use the information for the next 8 questions. In an experiment the reaction A+2B Products the following data were collected: A rate law has the form Rate =k[A]x[B]y where k is the rate constant, x is the order with respect to component A and y is the order with respect to component B. The square brackets around A and B indicate that these are concentrations. So [A] means the concentration of A. Looking at your two selected trials observe what happens to the rate when the concentration of A is doubled. If the rate does not change, we call this a zero order reaction for A and x=0. If the rate also doubles, we call this a first order reaction for A and x=1. If the rate quadruples, we call this a second order reaction for A and x=2. Based on the data. what is the order of the reaction for A? Explain your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


