Question: The molal boiling point elevation constant Kb=1.81Ckgmol1 for a certain substance X. When 86.39g of urea ((NH2) CO ) are dissolved in 950.g of X,
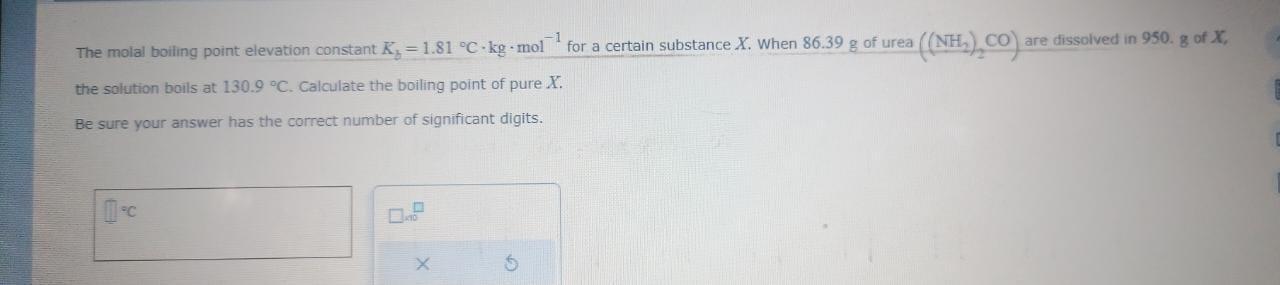
The molal boiling point elevation constant Kb=1.81Ckgmol1 for a certain substance X. When 86.39g of urea ((NH2) CO ) are dissolved in 950.g of X, the solution boils at 130.9C. Calculate the boiling point of pure X. Be sure your answer has the correct number of significant digits
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


