Question: The molecular partition function for a van der Waals gas can be written as: q = ne. (V Nb) exp[(-a. N/V)/kT] . (2) Here na
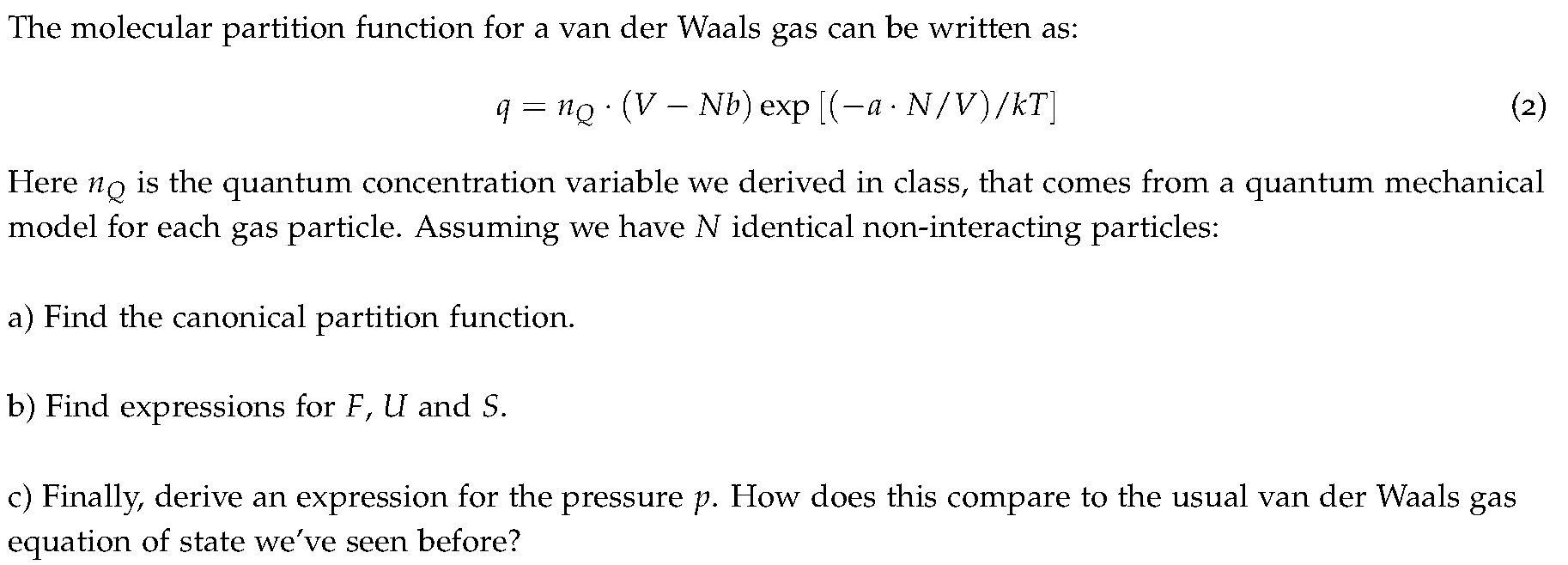
The molecular partition function for a van der Waals gas can be written as: q = ne. (V Nb) exp[(-a. N/V)/kT] . (2) Here na is the quantum concentration variable we derived in class, that comes from a quantum mechanical model for each gas particle. Assuming we have N identical non-interacting particles: a) Find the canonical partition function. b) Find expressions for F, U and S. c) Finally, derive an expression for the pressure p. How does this compare to the usual van der Waals gas equation of state we've seen before
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


