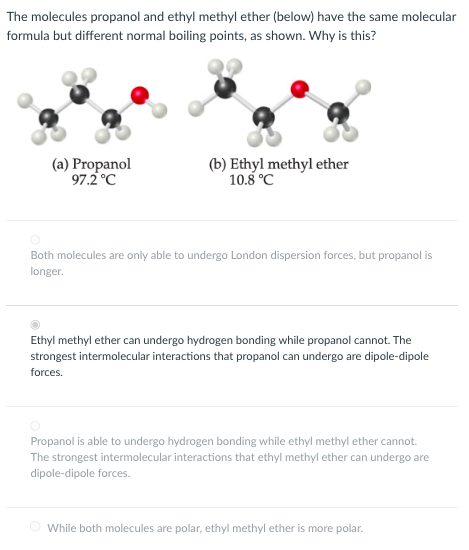
Question: The molecules propanol and ethyl methyl ether ( below ) have the same molecular formula but different normal boiling points, as shown. Why is this?
The molecules propanol and ethyl methyl ether below have the same molecular
formula but different normal boiling points, as shown. Why is this?
Both molecules are only able to undergo London dispersion forces, but propanol is
longer.
Ethyl methyl ether can undergo hydrogen bonding while propanol cannot. The
strongest intermolecular interactions that propanol can undergo are dipoledipole
forces.
Propanol is able to undergo hydrogen bonding while ethyl methyl ether cannot.
The strongest intermolecular interactions that ethyl methyl ether can undergo are
dipoledipole forces.
While both molecules are polar, ethyl methyl ether is more polar.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


