Question: The partial Lewis structure shown below is the carbon framework for a hydrocarbon molecule (containing only carbon and hydrogen): In the full Lewis structure, each
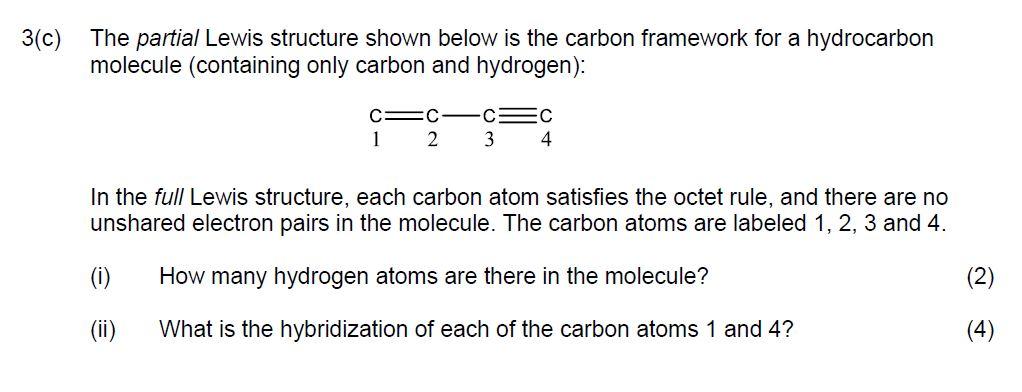
The partial Lewis structure shown below is the carbon framework for a hydrocarbon molecule (containing only carbon and hydrogen): In the full Lewis structure, each carbon atom satisfies the octet rule, and there are no unshared electron pairs in the molecule. The carbon atoms are labeled 1, 2, 3 and 4. (i) How many hydrogen atoms are there in the molecule? (ii) What is the hybridization of each of the carbon atoms 1 and 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


