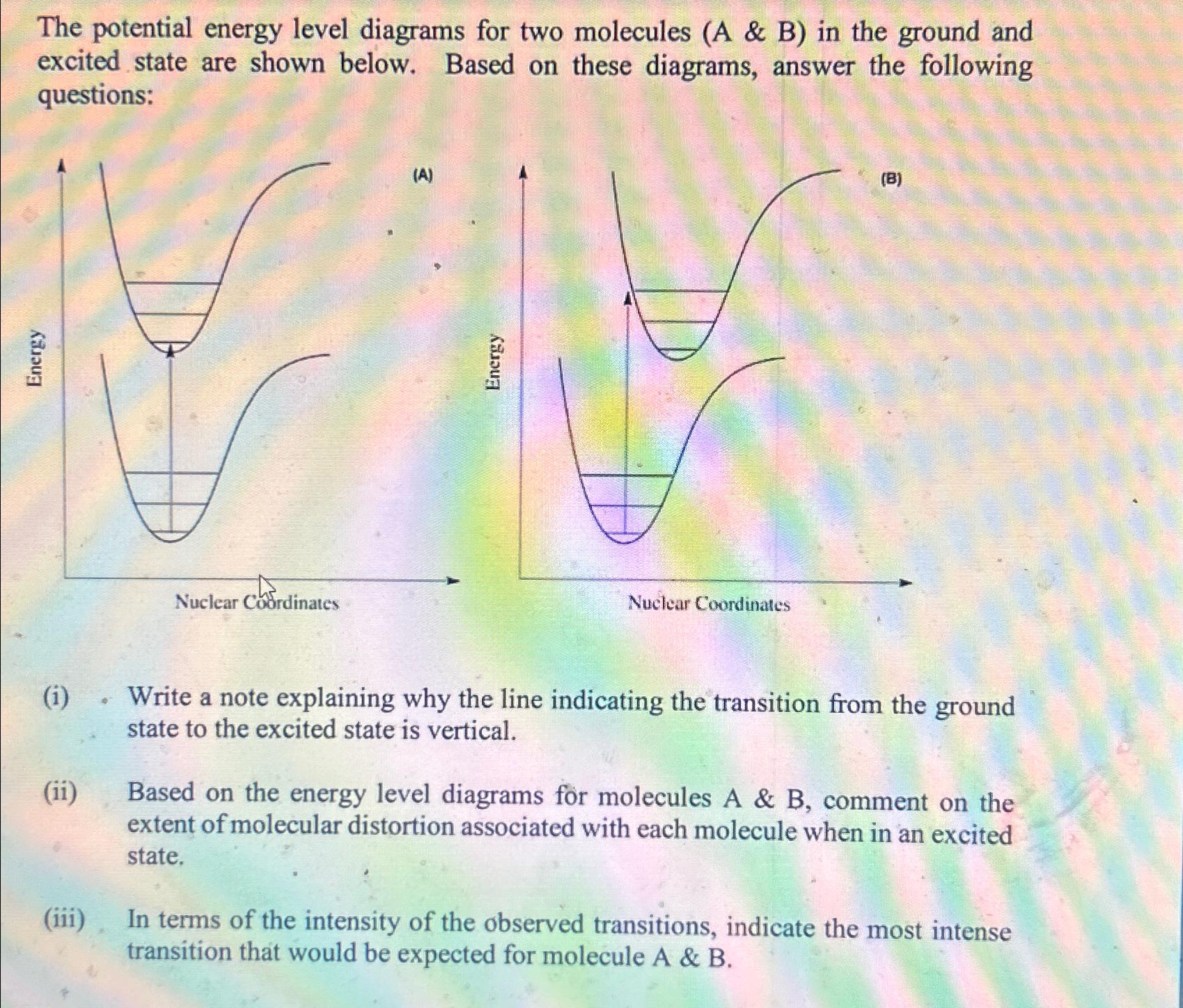
Question: The potential energy level diagrams for two molecules ( A & B ) in the ground and excited state are shown below. Based on these
The potential energy level diagrams for two molecules A & B in the ground and excited state are shown below. Based on these diagrams, answer the following questions:
i Write a note explaining why the line indicating the transition from the ground state to the excited state is vertical.
ii Based on the energy level diagrams for molecules A & B comment on the extent of molecular distortion associated with each molecule when in an excited state.
iii In terms of the intensity of the observed transitions, indicate the most intense transition that would be expected for molecule A & B
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


