Question: The question I need help with is question 5! Thank you Data Analysis: Show your work 1. How many mg of aspirin did you recover

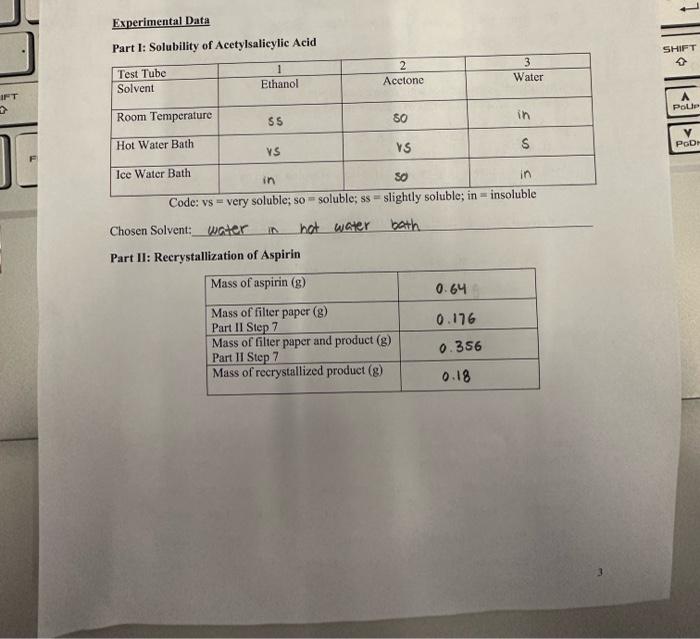
Data Analysis: Show your work 1. How many mg of aspirin did you recover from the enushed tablets (what was the mass of your recrystallized product)? 180mg of aspirn were recovered 2. How many mg of aspirin did you recover from each tablet? 90mg of aspiting from each tablet 3. What percent of the crushed tablets were pure aspirin? (Mass recovered / Initial Mass of tablets * 100% ) 0.640.18g100%=28% 4. Compare your answer in question 2 to the mass of aspirin listed on the bottle. What is the percent error in your experimental data compared to the actual mass of aspirin in each tablet? PercentError=Meastredmass(frombottle)|Measuredmass(frombottle)-Massyouoobtainedi100 5. Give 1 specific reason explaining your percent error in question 4. 6. Explain why you chose your recrystallization solvent. What propertics did this solvent have that made it the best for recrystallization? ve chose water as our solvent as t was the only solvent that was only soluble in the hot water bath Using acetane or ethanol wocld have ceused the recrystalization to fail before the last step in hot water. Part I: Solubility of Acetylsalicylic Acid Code: vs = very soluble; so = soluble; ss = shghtly soiuoie; in - ussuuvic Chosen Solvent: water in nat water bath Part II: Reerystallization of Aspirin
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


