Question: The rate constant k for a certain reaction is measured at two different temperatures: temperature 57.0 C 198.0 C E = Assuming the rate
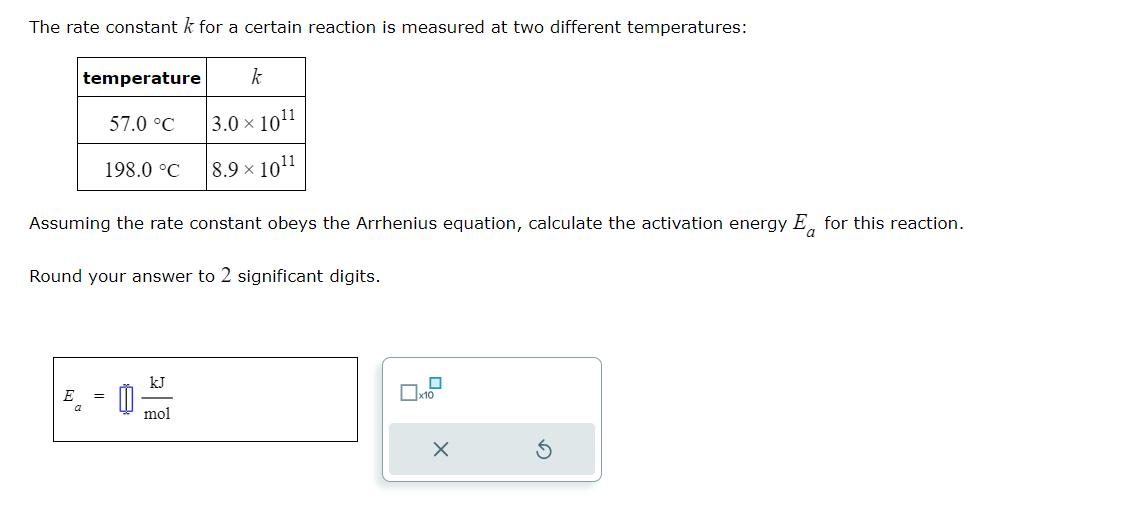
The rate constant k for a certain reaction is measured at two different temperatures: temperature 57.0 C 198.0 C E = Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E for this reaction. Round your answer to 2 significant digits. k 3.0101 8.9 x 101 kJ mol x10 X
Step by Step Solution
★★★★★
3.37 Rating (156 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Calculating Activation Energy Ea using Arrhenius Equation We can use the Arrhenius equation to find ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


