Question: PLEASE BE CORRECT and check your work Try Again Your answer is incorrect. The rate constant k for a certain reaction is measured at two
PLEASE BE CORRECT and check your work
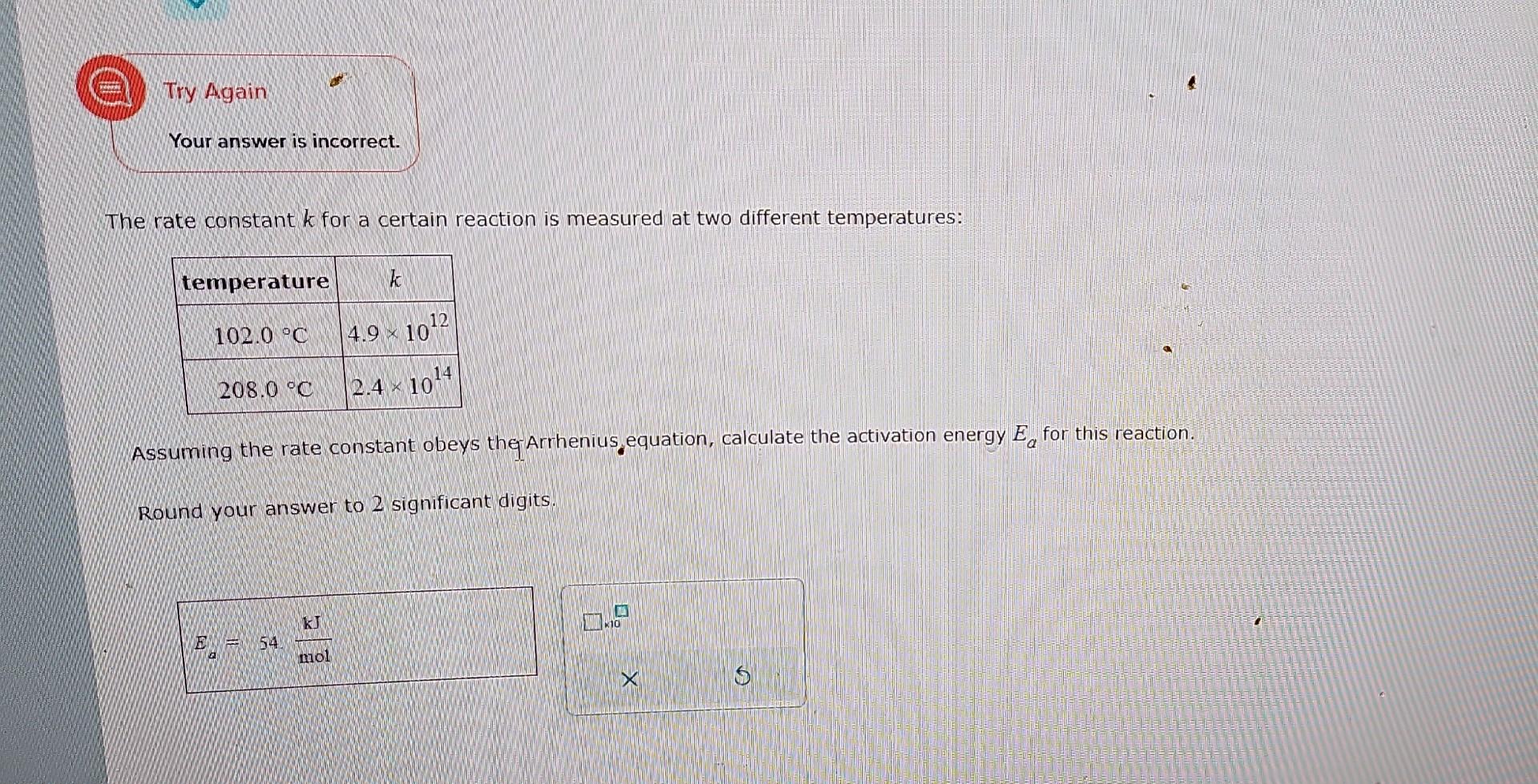
Try Again Your answer is incorrect. The rate constant k for a certain reaction is measured at two different temperatures: \begin{tabular}{|c|c|} \hline temperature & k \\ \hline 102.0C & 4.91012 \\ \hline 208.0C & 2.41014 \\ \hline \end{tabular} Assuming the rate constant obeys the Arrhenius, equation, calculate the activation energy Ea for this reaction. Round your answer to 2 significant digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


