Question: The rate data in Table 1 were obtained for the reaction of A+ S --> U. What is the order of the reaction with
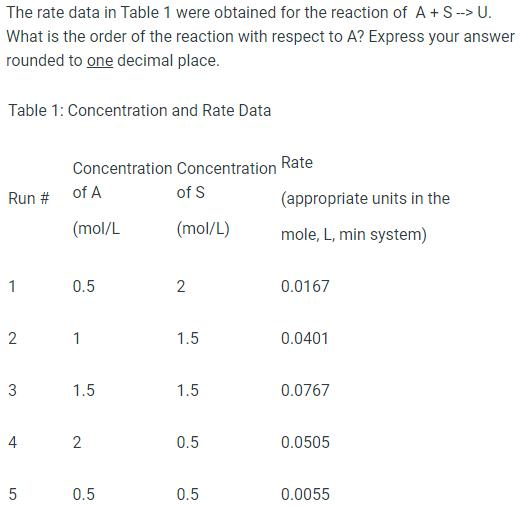
The rate data in Table 1 were obtained for the reaction of A+ S --> U. What is the order of the reaction with respect to A? Express your answer rounded to one decimal place. Table 1: Concentration and Rate Data Run # 1 2 3 4 5 Concentration of A (mol/L 0.5 1 1.5 2 0.5 Concentration of S (mol/L) 2 1.5 1.5 0.5 0.5 Rate (appropriate units in the mole, L, min system) 0.0167 0.0401 0.0767 0.0505 0.0055
Step by Step Solution
There are 3 Steps involved in it
To determine the order of the reaction with respect to reactant A we need to look at how changes in ... View full answer

Get step-by-step solutions from verified subject matter experts


