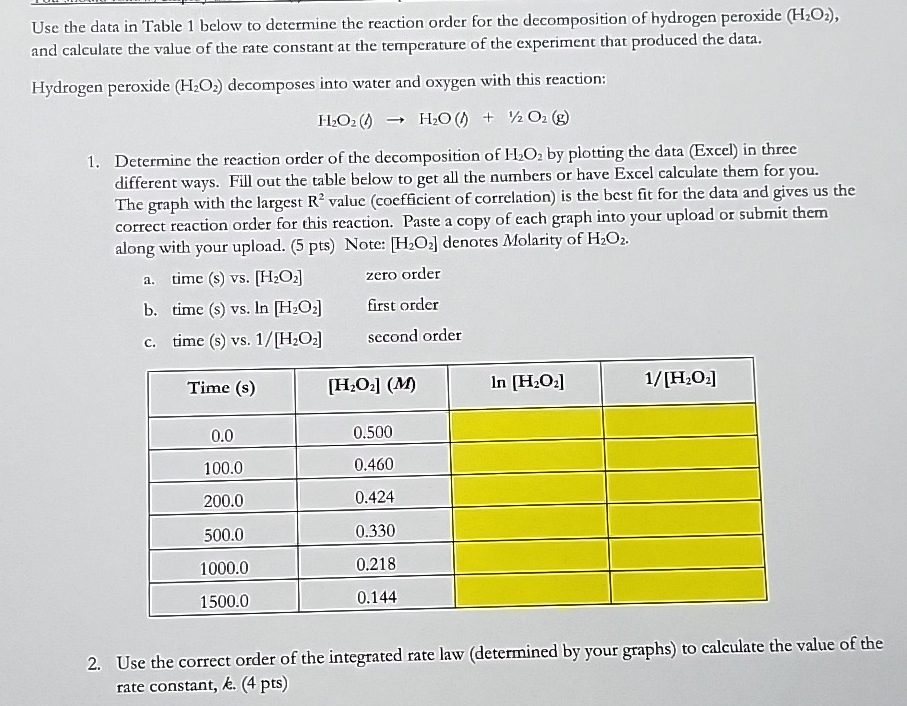
Question: Use the data in Table 1 below to determine the reaction order for the decomposition of hydrogen peroxide ( H 2 O 2 ) ,
Use the data in Table below to determine the reaction order for the decomposition of hydrogen peroxide and calculate the value of the rate constant at the temperature of the experiment that produced the data.
Hydrogen peroxide decomposes into water and oxygen with this reaction:
Determine the reaction order of the decomposition of by plotting the data Excel in three different ways. Fill out the table below to get all the numbers or have Excel calculate them for you. The graph with the largest value coefficient of correlation is the best fit for the data and gives us the correct reaction order for this reaction. Paste a copy of each graph into your upload or submit them along with your upload. pts Note: denotes Molarity of
a time s vs zero order
b time s vs first order
c time s vs second order
tableTime s
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


