Question: The reaction 3A + 2B P has a second-order rate law v = k [A] [B]. The initial molar concentrations of A and B are
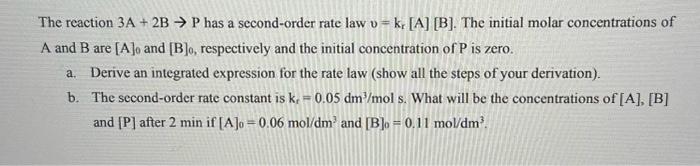
The reaction 3A+2BP has a second-order rate law v=kr[A][B]. The initial molar concentrations of A and B are [A]0 and [B]0, respectively and the initial concentration of P is zero. a. Derive an integrated expression for the rate law (show all the steps of your derivation). b. The second-order rate constant is kr=0.05dm3/mols. What will be the concentrations of [A], [B] and [P] after 2min if [A]0=0.06mol/dm3 and [B]0=0.11mol/dm3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


