Question: The reaction 4 HBr(9) + O2(9) -> 2H2O(g) + 2 Br(a) proceeds through the following mechanism: HBr(9) + Oy(9) --> HOOBr(9) HOOB (9) + HBr
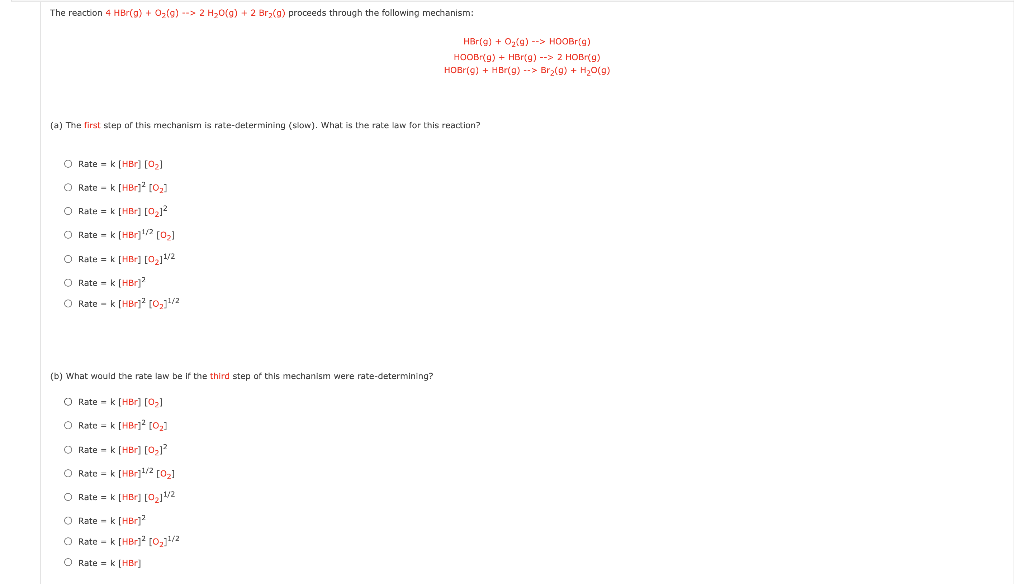
The reaction 4 HBr(9) + O2(9) -> 2H2O(g) + 2 Br(a) proceeds through the following mechanism: HBr(9) + Oy(9) --> HOOBr(9) HOOB (9) + HBr (9) --> 2 HOB (9) HOB(9) + HBr(9) --> Br2(9) + H20(9) (a) The first step of this mechanism is rate-determining (slow). What is the rate law for this reaction? O Rate -k (HBr) (0) Rate - k [Br] [0,] O Rate = k [HB] [O212 O Rate -k (HBr)? (0) O Rate = k [Br] [0,1/2 O Rate - k (HBr)' Rate - k [HBr]2[0,1/2 (b) What would the rate law be if the third step of this mechanism were rate-determining? O Rate -k (HBr) (0,1 O Rate = k (HBr] 0,3 O Rate - k (HBr] (0,1 O Rate = k [Br]1/2 [02] O Rate = k [HBr] [2]1/2 O Rate -k (HBr? O Rate - k [HBri2 [0221/2 O Rate = k (HB)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


