Question: The reaction: A ? 2 ? K C + - darr With reaction kinetics: r A = K C A C B 3 , andr
The reaction: darr With reaction kinetics:
andr
occurs in a constant Volume Batch Reactor.
Obtain the concentration of the reactants after omins if they were initially charged at equimolar
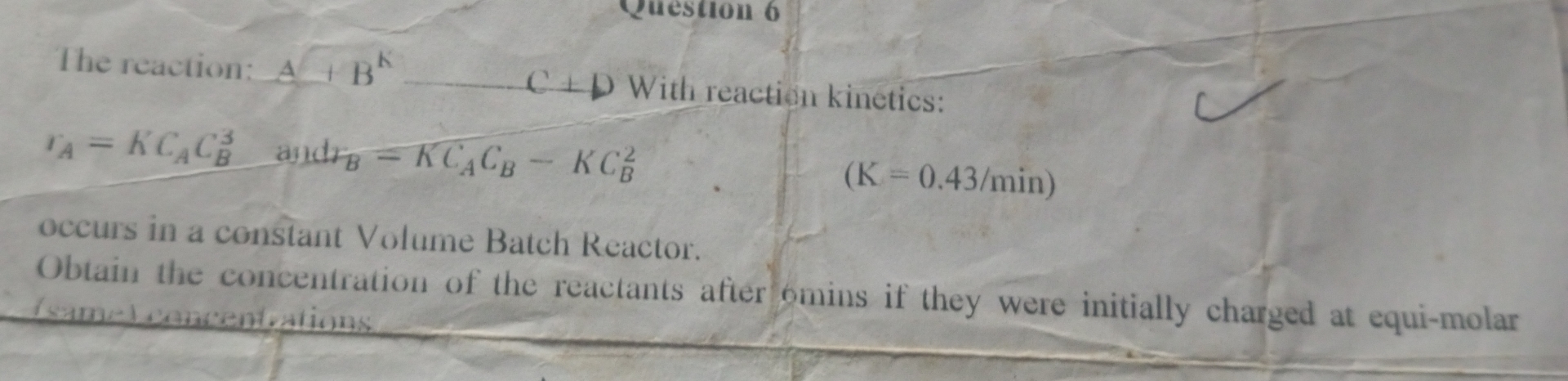
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


