Question: Please help ASAP 2. Given in the table below are the standard enthalpy of formation and entropy values for the species in the following chemical
Please help ASAP
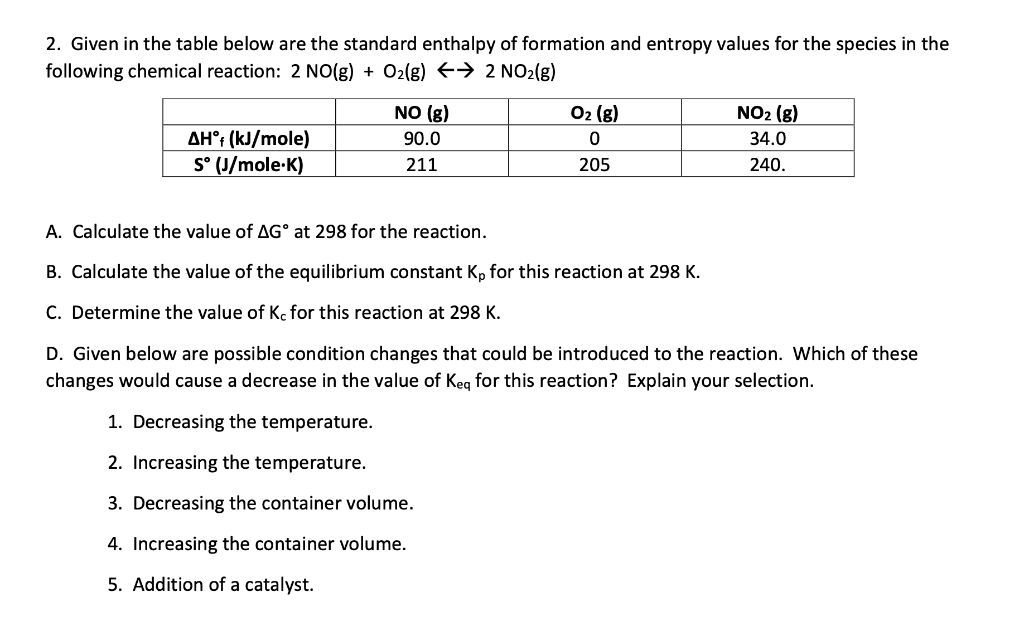
2. Given in the table below are the standard enthalpy of formation and entropy values for the species in the following chemical reaction: 2 NO(g) + O2(g) 2 NO2(g) AHt (kJ/mole) S (J/mole.K) NO (g) 90.0 211 O2 (g) 0 205 NO2 (g) 34.0 240. A. Calculate the value of AG at 298 for the reaction. B. Calculate the value of the equilibrium constant Kp for this reaction at 298 K. C. Determine the value of Kc for this reaction at 298 K. D. Given below are possible condition changes that could be introduced to the reaction. Which of these changes would cause a decrease in the value of Keq for this reaction? Explain your selection. 1. Decreasing the temperature. 2. Increasing the temperature. 3. Decreasing the container volume. 4. Increasing the container volume. 5. Addition of a catalyst
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


