Question: The reaction below was studied at constant temperature with the following results: 6. (8 points) The reaction below was studied at constant temperature with the
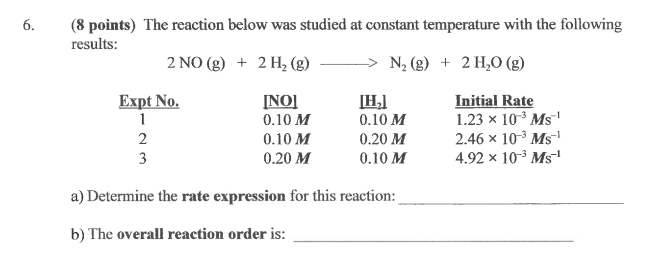 The reaction below was studied at constant temperature with the following results:
The reaction below was studied at constant temperature with the following results:
6. (8 points) The reaction below was studied at constant temperature with the following results: 2 NO(g) + 2 H, (g) N2(g) + 2 H20 (g) Expt No. [H] Initial Rate 1 0.10 M 0.10 M 1.23 x 10 Ms 2 0.10 M 0.20 M 2.46 x 10-3Ms 3 0.20 M 0.10 M 4.92 x 10- Ms! a) Determine the rate expression for this reaction: b) The overall reaction order is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


