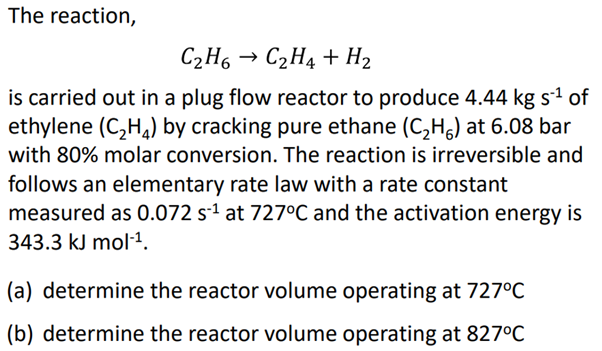
Question: The reaction, C 2 H 6 C 2 H 4 + H 2 is carried out in a plug flow reactor to produce 4 .
The reaction,
is carried out in a plug flow reactor to produce of
ethylene by cracking pure ethane at bar
with molar conversion. The reaction is irreversible and
follows an elementary rate law with a rate constant
measured as at and the activation energy is
a determine the reactor volume operating at
b determine the reactor volume operating at
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


