Question: The reaction given below was performed in a batch reactor and in the gas phase. 10% N2 was introduced in the reactor at 2atm and
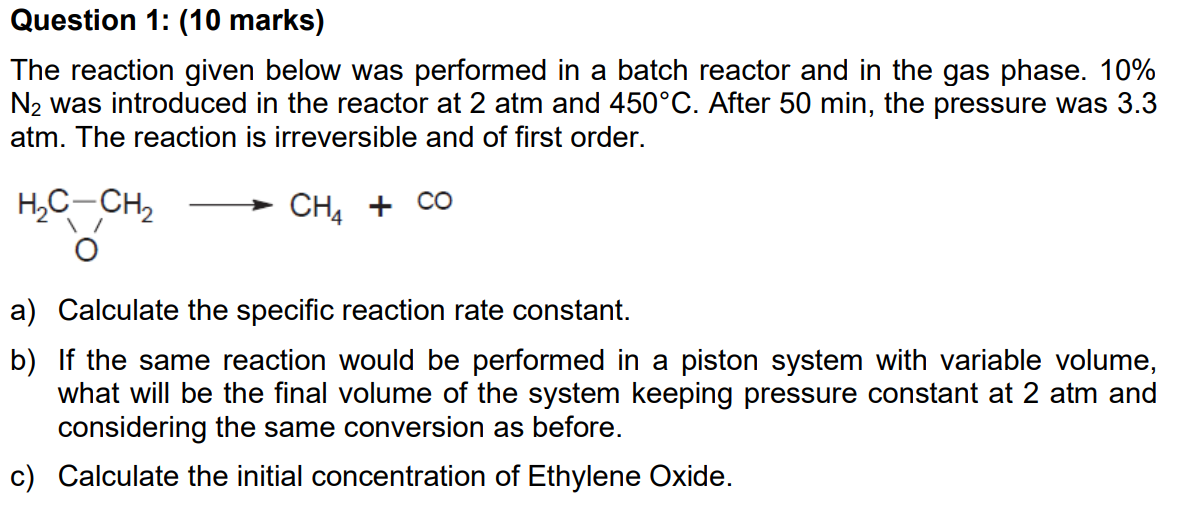
The reaction given below was performed in a batch reactor and in the gas phase. 10\% N2 was introduced in the reactor at 2atm and 450C. After 50min, the pressure was 3.3 atm. The reaction is irreversible and of first order. a) Calculate the specific reaction rate constant. b) If the same reaction would be performed in a piston system with variable volume, what will be the final volume of the system keeping pressure constant at 2atm and considering the same conversion as before. c) Calculate the initial concentration of Ethylene Oxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


