Question: The reaction of C2H6 with oxygen produces carbon dioxide and H20 according to the following reaction: C2H6 + 02 CO2 + H20 Balance the
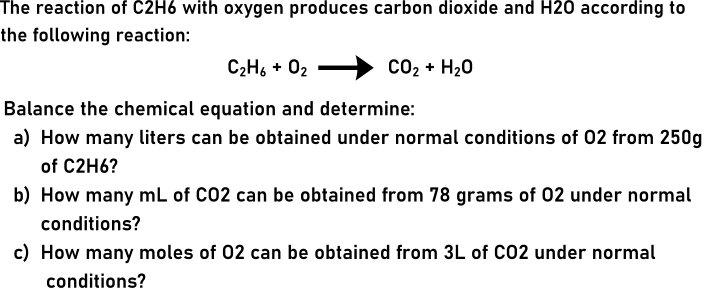
The reaction of C2H6 with oxygen produces carbon dioxide and H20 according to the following reaction: C2H6 + 02 CO2 + H20 Balance the chemical equation and determine: a) How many liters can be obtained under normal conditions of 02 from 250g of C2H6? b) How many mL of C02 can be obtained from 78 grams of 02 under normal conditions? c) How many moles of 02 can be obtained from 3L of C02 under normal conditions?
Step by Step Solution
There are 3 Steps involved in it
Balanced reaction C2 Ho 702 202 3H20 2 a Given Oz is in excess ... View full answer

Get step-by-step solutions from verified subject matter experts


