Question: The reaction: Type numbers in the boxes. is found to have the rate law: 3A+2B+Cproducts 'Part 1: 2 points ' Part 2: 2 points 'Part
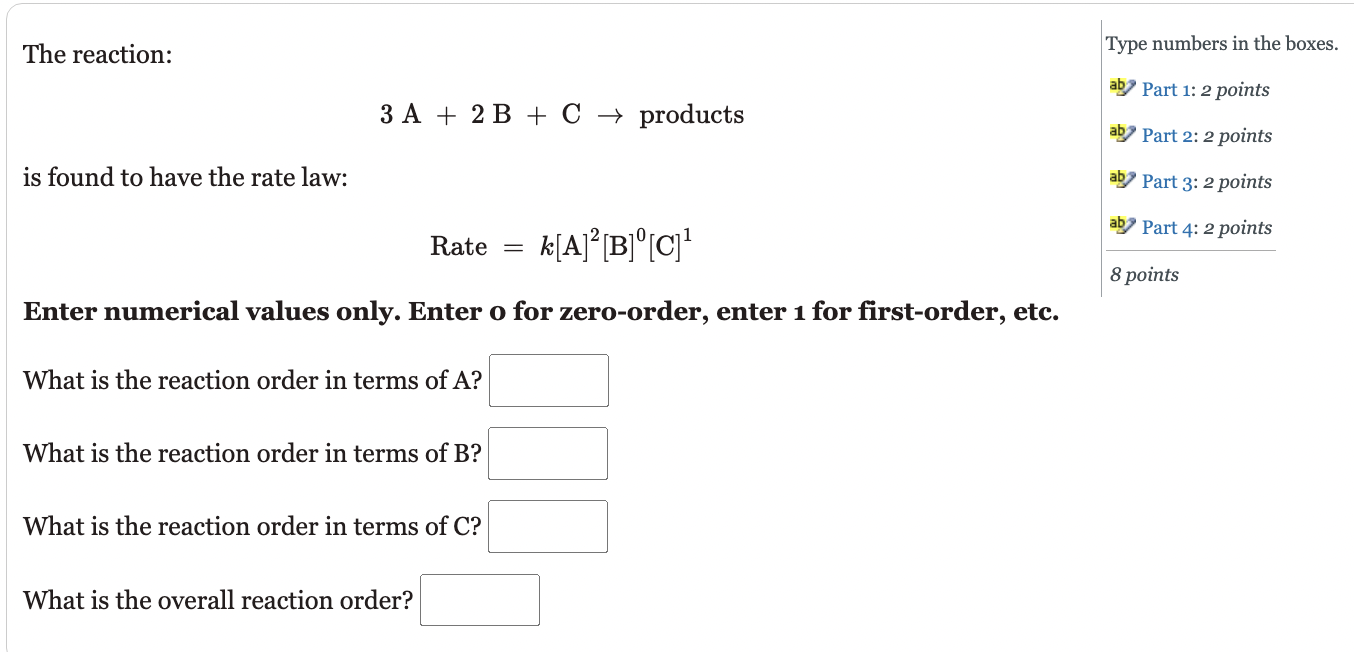
The reaction: Type numbers in the boxes. is found to have the rate law: 3A+2B+Cproducts 'Part 1: 2 points ' Part 2: 2 points 'Part 3: 2 points Rate=k[A]2[B]0[C]1 Enter numerical values only. Enter o for zero-order, enter 1 for first-order, etc. What is the reaction order in terms of A ? What is the reaction order in terms of B ? What is the reaction order in terms of C? What is the overall reaction order
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


