Question: Question 2 Select one answer. Given the following reaction, 5 points 2 NH3 (g) + N2(g) + 3H2 (9) which of the following relates the
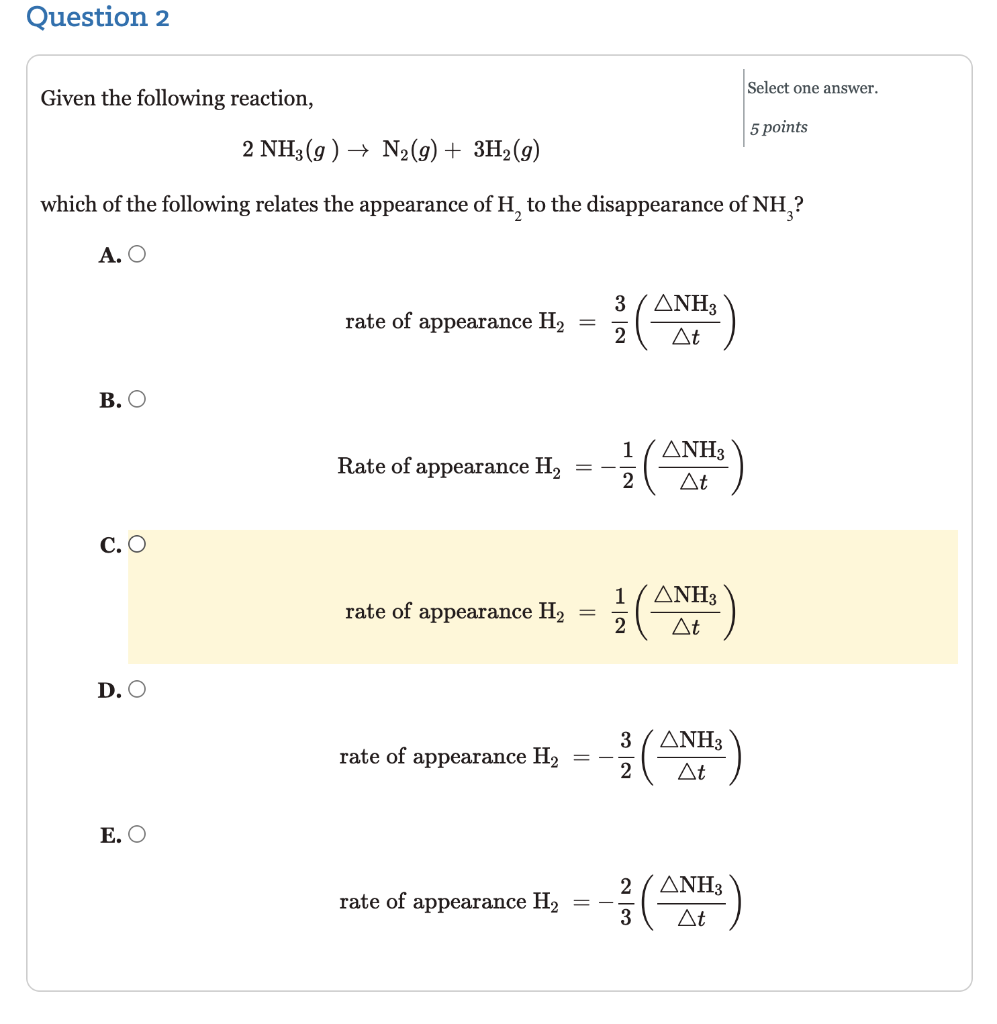
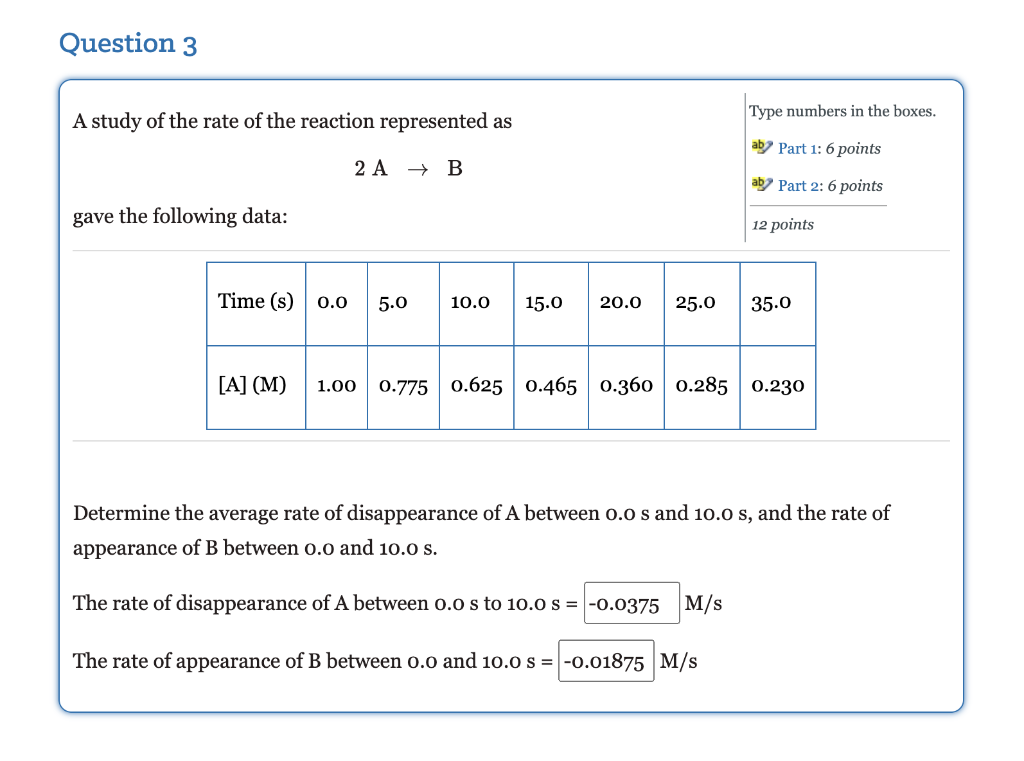
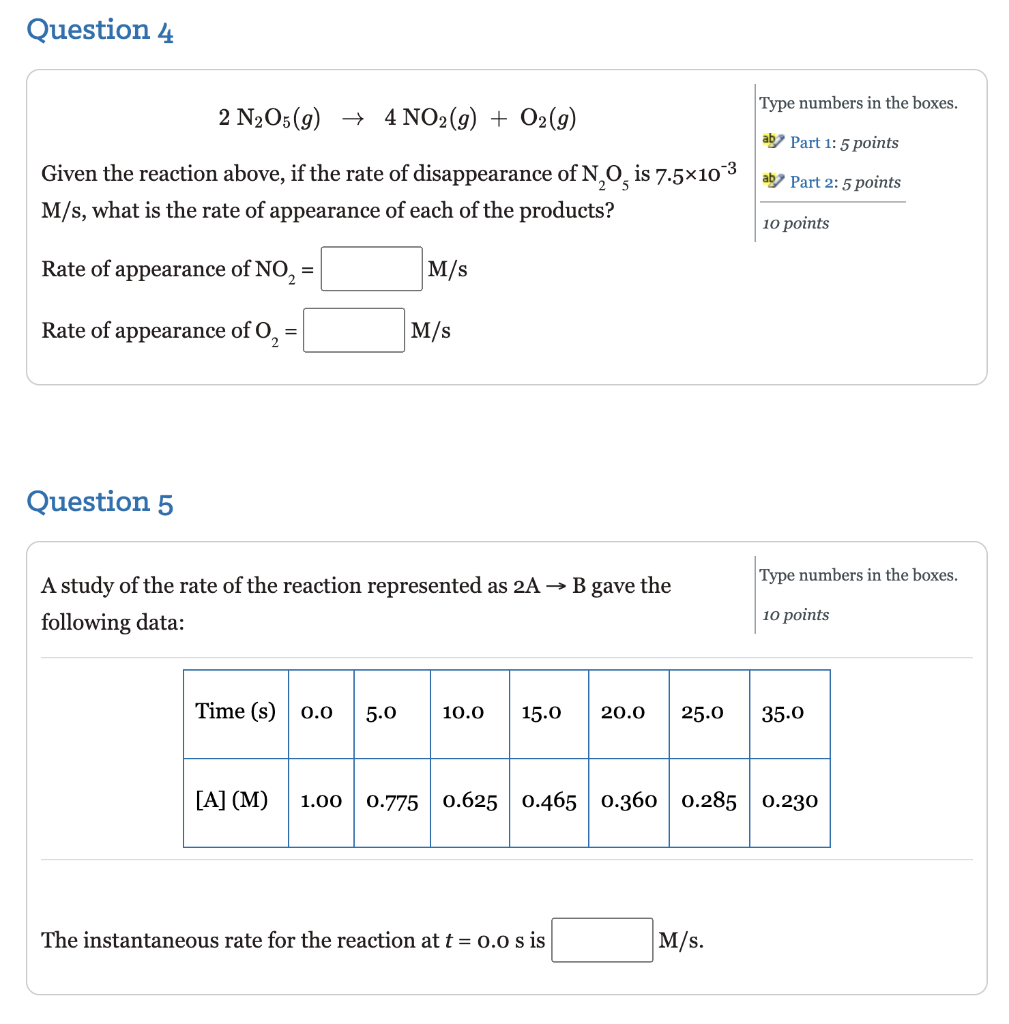
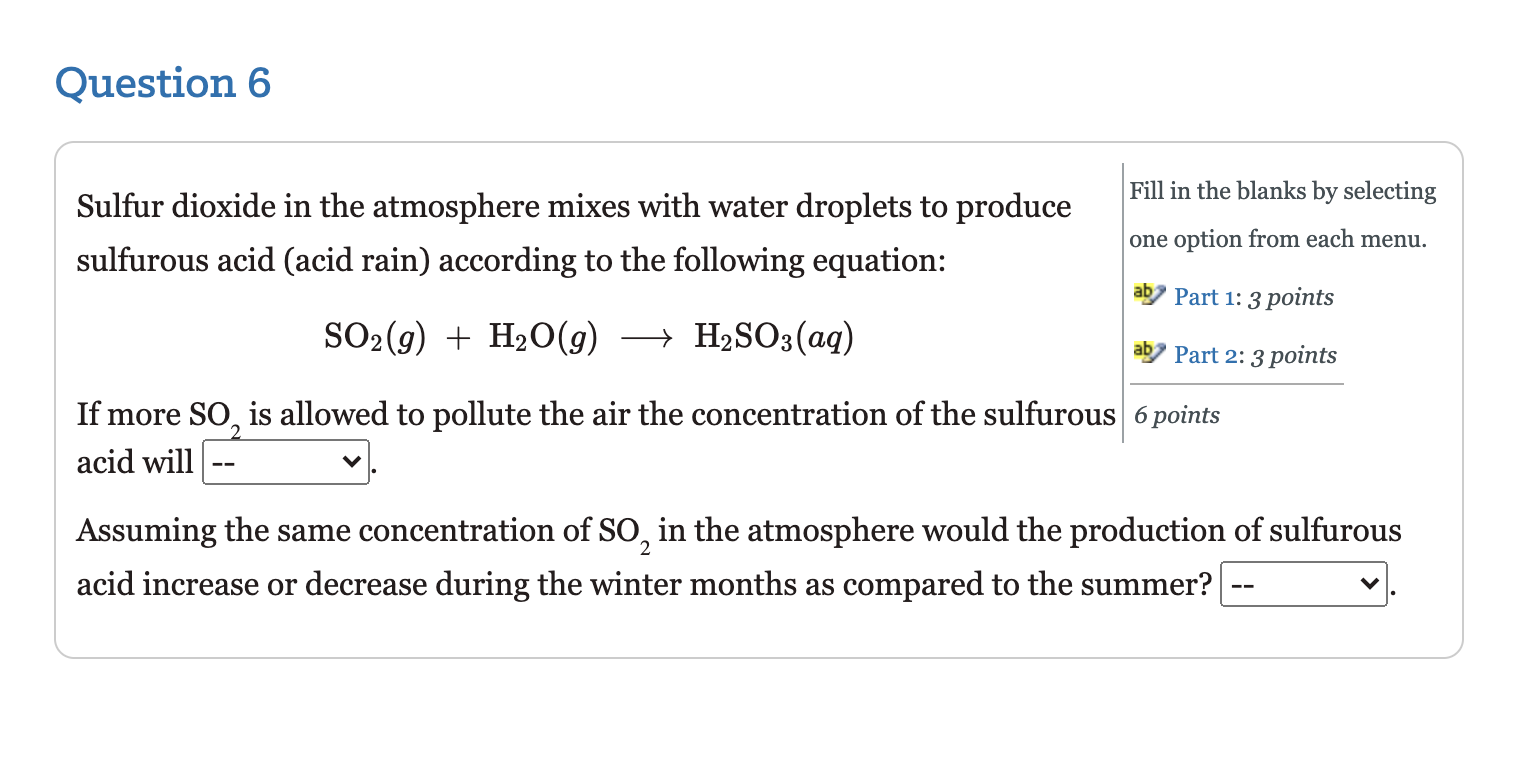
Question 2 Select one answer. Given the following reaction, 5 points 2 NH3 (g) + N2(g) + 3H2 (9) which of the following relates the appearance of H, to the disappearance of NH,? . rate of appearance H2 37 3 2 t . 1 Rate of appearance H2 - - 3 t 2 C. ( rate of appearance H2 = 1 2 3 t D. 3 rate of appearance H, 3 t 2 . 2 rate of appearance H2 3 3 t 3 Question 3 A study of the rate of the reaction represented as Type numbers in the boxes. ab Part 1: 6 points 2 A + B ab Part 2: 6 points gave the following data: 12 points Time (s) 0.0 5.0 10.0 15.0 20.0 25.0 35.0 [A] (M) 1.00 0.775 0.625 0.465 0.360 0.285 0.230 Determine the average rate of disappearance of A between o.o s and 10.0 s, and the rate of appearance of B between o.o and 10.0 s. The rate of disappearance of A between 0.o s to 10.0 S = -0.0375 M/s The rate of appearance of B between o.o and 10.0 s= -0.01875 M/s Question 4 Type numbers in the boxes. 2 N2O5(g) + 4 NO2(g) + O2(g) aby Part 1: 5 points Given the reaction above, if the rate of disappearance of N, O, is 7.5x10 3 ad Part 2: 5 points M/s, what is the rate of appearance of each of the products? 10 points Rate of appearance of NO2 M/s Rate of appearance of O, M/s Question 5 Type numbers the boxes. A study of the rate of the reaction represented as 2A B gave the following data: 10 points Time (s) 0.0 5.0 10.0 15.0 20.0 25.0 35.0 [A] (M) 1.00 0.775 0.625 0.465 0.360 0.285 0.230 The instantaneous rate for the reaction at t = 0.0 s is M/s. Question 6 Sulfur dioxide in the atmosphere mixes with water droplets to produce sulfurous acid (acid rain) according to the following equation: Fill in the blanks by selecting one option from each menu. aby Part 1: 3 points SO2(g) + H2O(g) + H2SO3(aq) Part 2: 3 points If more So, is allowed to pollute the air the concentration of the sulfurous 6 points acid will Assuming the same concentration of So, in the atmosphere would the production of sulfurous acid increase or decrease during the winter months as compared to the summer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


