Question: The selectivity coefficient, KK., for at ion-selective electrode is 0.00649. When the electrode is placed into a 5.01 x 10-5 M K+ solution at pH
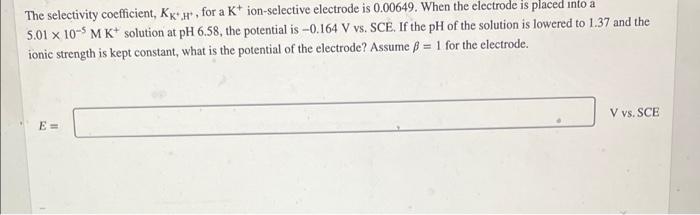
The selectivity coefficient, KK., for at ion-selective electrode is 0.00649. When the electrode is placed into a 5.01 x 10-5 M K+ solution at pH 6.58, the potential is -0.164 V vs. SCE. If the pH of the solution is lowered to 1.37 and the ionic strength is kept constant, what is the potential of the electrode? Assume B = 1 for the electrode. V vs. SCE E
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


