Question: The standard cell potential E for the reaction at 25C Fe+Zn2+=Zn+Fe2+ is 0.353V. If a piece of iron is placed in a 1MZn2+ solution, what
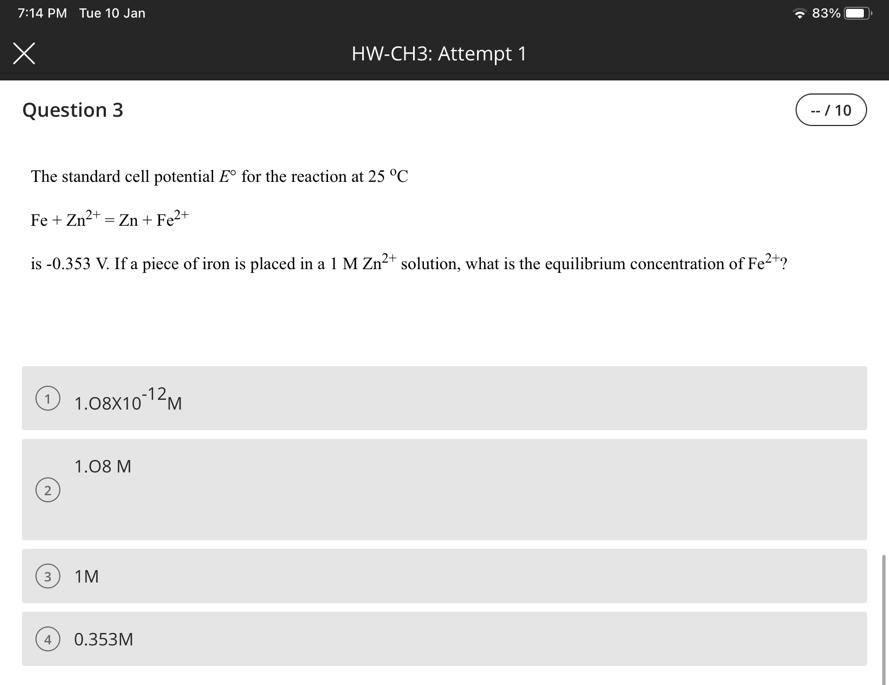
The standard cell potential E for the reaction at 25C Fe+Zn2+=Zn+Fe2+ is 0.353V. If a piece of iron is placed in a 1MZn2+ solution, what is the equilibrium concentration of Fe2+ ? 1.081012M 1.08M 1M 0.353M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


