Question: (i) The number of moles of O2(g) that was produced (ii) The mass ofH2O2(aq)that decomposed (d) The student continues the experiment for an additional minute.
(i) The number of moles of O2(g) that was produced
(ii) The mass of H2O2(aq) that decomposed
(d) The student continues the experiment for an additional minute. For beaker 2, will the mass of H2O2(aq) consumed during the second minute be greater than, less than, or equal to the mass of H2O2(aq) consumed during the first minute? Explain your answer referring to the data in the table.
(e) Based on the data, the student claims that the catalyzed reaction has zeroth-order kinetics. Do you agree with the student’s claim? Justify your answer.
(f) A second student did the experiment using larger volumes of 9.77MH2O2(aq) . The student noticed that the reaction in beaker 2 proceeded extremely rapidly, causing some of the liquid to splash out of the beaker onto the lab table. The student claims that as a result of the loss of the liquid from the beaker, the calculated number of moles of O2(g) produced is greater than the actual number of moles of O2(g) produced during the first 60 seconds. Do you agree with the student? Justify your answer.
(g) The hydrogen peroxide used in this experiment can be prepared by the reaction of solid ammonium peroxydisulfate, (NH4)2S2O8, with water. The products are hydrogen peroxide (H2O2) and ammonium bisulfate (NH4HSO4). Write the balanced equation for the reaction.
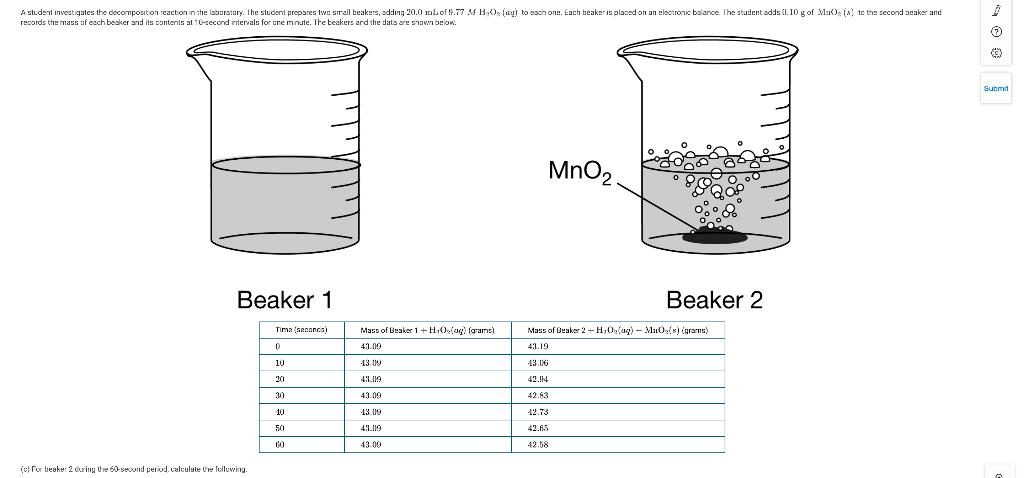
A student investigates the decomposition reaction in the labaratury. The student prepares twe srall beakers, adding 20.0 mLof 9.77 M H,Os (agl to each one. Each beaker is placed on an electronic balance. The student adds (l.10 g of Ma)s (A tc the second beaker and records the mass of cach beaker and its contents at 10-second ntervals for cre mnute. The beakers and the data are shown below. Submit MnO2 Beaker 1 Beaker 2 Tima (secanca) Mass of Beaker 1+ H,Os(ag) (grams) Mass of Braker 2H,O(ag) - Mu0(s) (grams) 43.09 43.19 10 13. 09 43.06 20 43.19 42.14 30 43.00 42.83 13.09 12.73 43.09 42.65 60 43.00 42.58 (e) For beaker 2 during Uhe 60-second period, calkulate Ure fellewing.
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
1 00190625 mol O 2 129686 g H 2 O 2 2 Yes the 2nd minute will will consume less mass of H 2 O 2 3No ... View full answer

Get step-by-step solutions from verified subject matter experts


