Question: The table below shows data obtained at 25C for the theoretical reaction represented by the balanced equation: 2 A + B + 2 Ca 3D
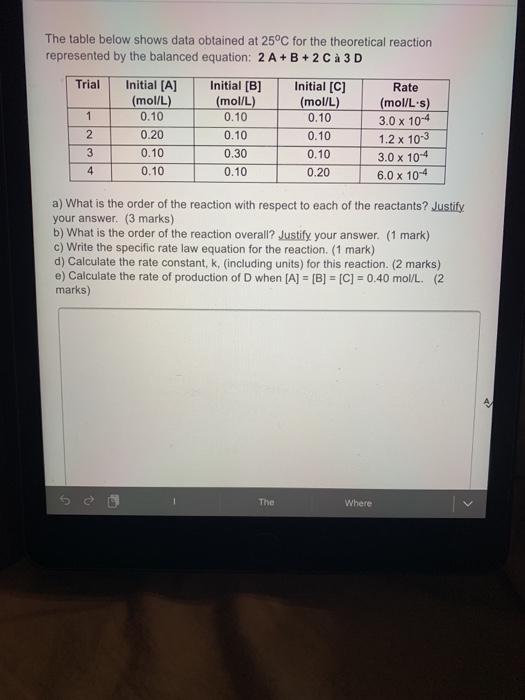
The table below shows data obtained at 25C for the theoretical reaction represented by the balanced equation: 2 A + B + 2 Ca 3D Trial Initial [B] (mol/L) 1 0.10 Initial [A] (mol/L) 0.10 0.20 0.10 0.10 2 Initial [C] (mol/L) 0.10 0.10 0.10 0.20 0.10 0.30 0.10 Rate (mol/L.s) 3.0 x 10-4 1.2 x 10-3 3.0 x 10-4 6.0 x 10-4 3 4 a) What is the order of the reaction with respect to each of the reactants? Justify your answer. (3 marks) b) What is the order of the reaction overall? Justify your answer. (1 mark) c) Write the specific rate law equation for the reaction. (1 mark) d) Calculate the rate constant, k. (including units) for this reaction. (2 marks) e) Calculate the rate of production of D when [A] = [B] = [C] = 0.40 mol/L. (2 marks) The Where >
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


