Question: The table gives the concentrations of C2H4O(g) as a function of time at a certain temperature for the reaction shown. C2H4O(g)CH4(g)+CO(g) Verify that this is
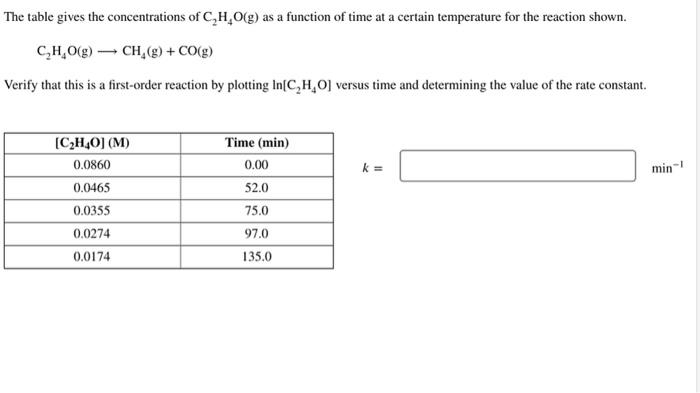
The table gives the concentrations of C2H4O(g) as a function of time at a certain temperature for the reaction shown. C2H4O(g)CH4(g)+CO(g) Verify that this is a first-order reaction by plotting ln[C2H4O] versus time and determining the value of the rate constant
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


