Question: The temperature of the above equilibrium system is increased while kept at a constant volume. A new state of equilibrium is established in which there
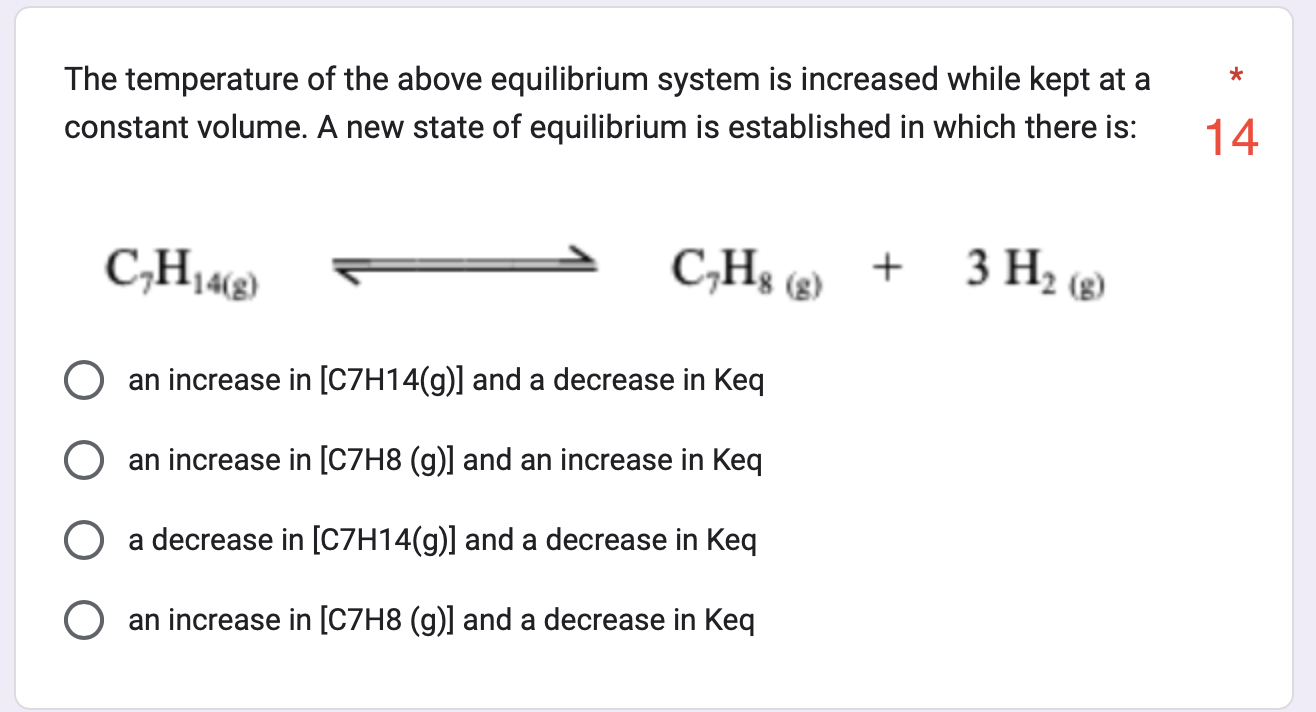
The temperature of the above equilibrium system is increased while kept at a constant volume. A new state of equilibrium is established in which there is: an increase in [C7H14(g)] and a decrease in Keq an increase in [C7H8 (g)] and an increase in Keq a decrease in [C7H14(g)] and a decrease in Keq an increase in [C7H8 (g)] and a decrease in Ke
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


