Question: both pleasee Write the equilibrium constant expression, K, for the following reaction: If either the numerator or denominator is blank, please enter 1 CoCO3(s)Co2+(aq)+CO32(aq) 9
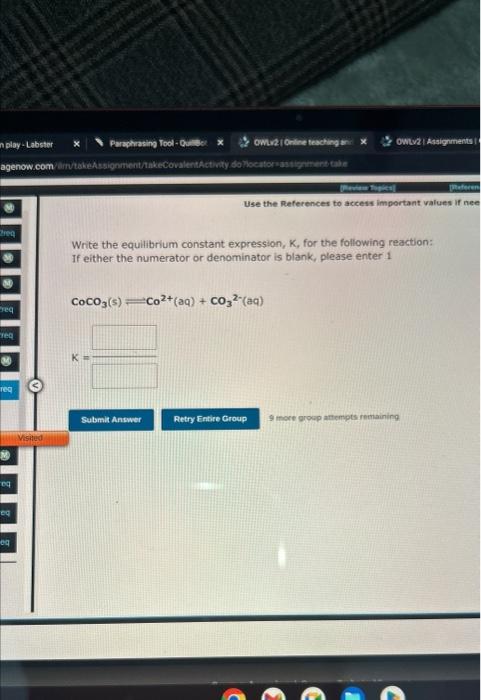
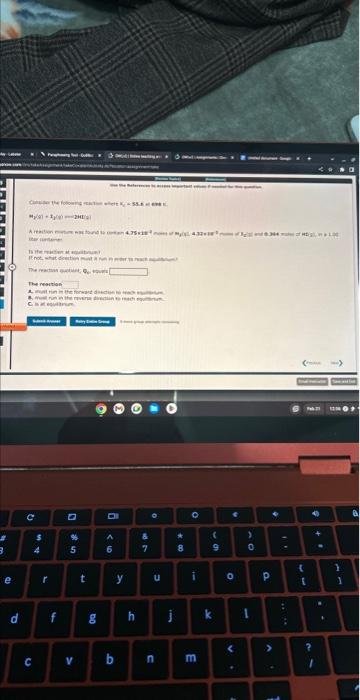
Write the equilibrium constant expression, K, for the following reaction: If either the numerator or denominator is blank, please enter 1 CoCO3(s)Co2+(aq)+CO32(aq) 9 inert growp attempots remaining My(e) +xY rit =241D:= The reartion C. it it esialrem. Write the equilibrium constant expression, K, for the following reaction: If either the numerator or denominator is blank, please enter 1 CoCO3(s)Co2+(aq)+CO32(aq) 9 inert growp attempots remaining My(e) +xY rit =241D:= The reartion C. it it esialrem
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


