Question: The water-gas shift reaction is given as CO + H2O=CO2 + H2 Under certain operating conditions the equilibrium constant for this reaction has the following
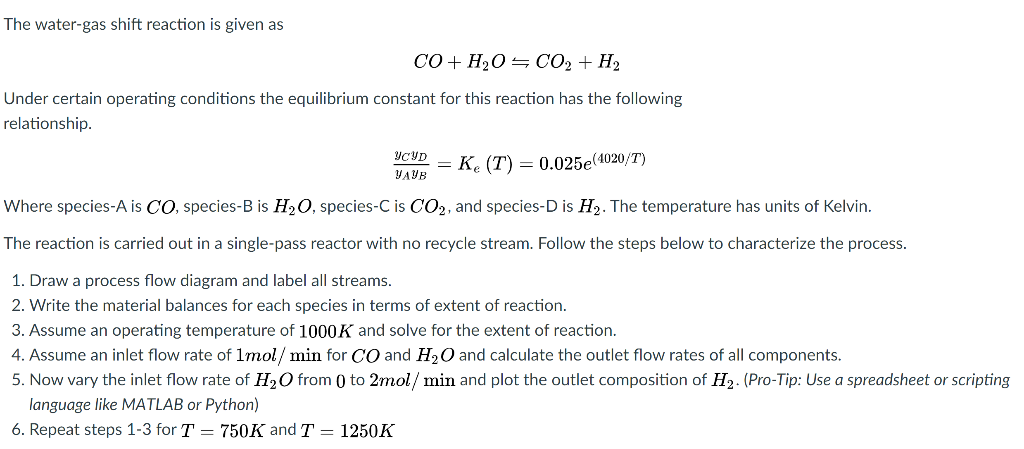
The water-gas shift reaction is given as CO + H2O=CO2 + H2 Under certain operating conditions the equilibrium constant for this reaction has the following relationship. YAYB = Ke (T) = 0.025e(4020/T) Where species-A is CO, species-B is H2O, species-C is CO2, and species-D is H. The temperature has units of Kelvin. The reaction is carried out in a single-pass reactor with no recycle stream. Follow the steps below to characterize the process. 1. Draw a process flow diagram and label all streams. 2. Write the material balances for each species in terms of extent of reaction. 3. Assume an operating temperature of 1000K and solve for the extent of reaction. 4. Assume an inlet flow rate of Imol/ min for CO and H2O and calculate the outlet flow rates of all components. 5. Now vary the inlet flow rate of H2O from 0 to 2mol/ min and plot the outlet composition of H, (Pro-Tip: Use a spreadsheet or scripting language like MATLAB or Python) 6. Repeat steps 1-3 for T = 750K and T = 1250K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


