Question: Theoretical density is 1.190 g/mL (10pts) Percent Error Ask your instructor for the theoretical density of your unknown liquid. Enter it below thon cakculate the
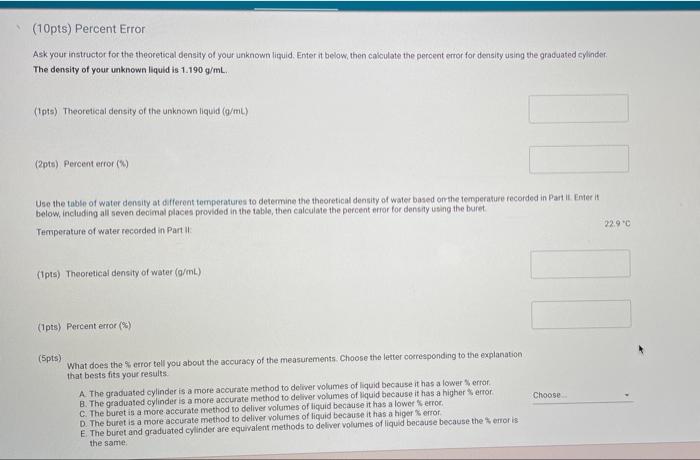
Theoretical density is 1.190 g/mL
(10pts) Percent Error Ask your instructor for the theoretical density of your unknown liquid. Enter it below thon cakculate the percent error for density using the graduated cylinder, The density of your unknown liquid is 1.190 g/mL. (pt) Theoretical density of the unknown liquid (9/mL) (2pts) Percent error (%) Use the table of water density at different temperatures to determine the theoretical density of water based on the temperature recorded in Partit Enterit below, including all seven decimal places provided in the table, then calculate the percent error for density using the buret Temperature of water recorded in Parti 22.90 (Ipts) Theoretical density of water (/m) (Ipts) Percent error (*) (Spts) Choose What does the error tell you about the accuracy of the measurements. Choose the letter corresponding to the explanation that best fits your results A The graduated cylinder is a more accurate method to deliver volumes of liquid because it has a lower error B. The graduated cylinder is a more accurate method to deliver volumes of liquid because it has a higher error C. The buret is a more accurate method to deliver volumes of liquid because it has a lower error D. The buret is a more accurate method to deliver volumes of liquid because it has a higer error E The buret and graduated cylinder are equivalent methods to deliver volumes of liquid because because the error is the same
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


