Question: There are conventions for naming and constructing the formulas for acids and bases just as there are for other types of compounds. There are
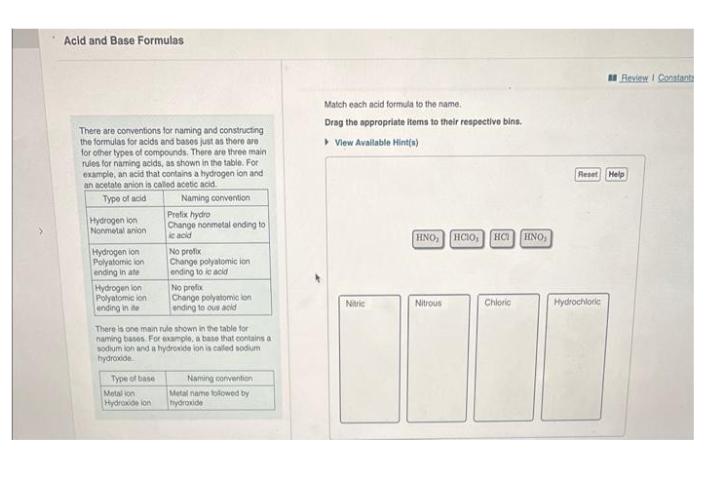
There are conventions for naming and constructing the formulas for acids and bases just as there are for other types of compounds. There are three main rules for naming acids, as shown in the table. For example, an acid that contains a hydrogen ion and an acetate anion is called acetic acid. Type of acid Hydrogen lon Nonmetal anion Hydrogen ion Polyatomic ion ending in ate Hydrogen lon Polyatomic ion ending in the Type of base Naming convention Metal ion Hydroxide ion Prefix hydro Change nonmetal ending to ic acid No profix Change polyatomic ion ending to ic acid There is one main rule shown in the table for naming bases For example, a base that contains a sodium ion and a hydroxide ion is called sodium hydroxide No prefix Change polyatomic lon ending to ous acid Naming convention Metal name followed by hydroxide Match each acid formula to the name. Drag the appropriate items to their respective bins. View Available Hint(s) Nitric HNO Nitrous HCIO, HC Chloric HNO, Review I Constant Reset Help Hydrochloric
Step by Step Solution
3.28 Rating (148 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


