Question: There is a reaction between solid copper and nitric acid: A. Use the data in the following table and calculate the standard courtship potential for
There is a reaction between solid copper and nitric acid:
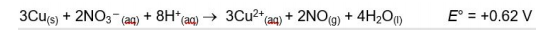
A. Use the data in the following table and calculate the standard courtship potential for half The redox reaction of - (NO3aq) in acidic solution. Show calculations
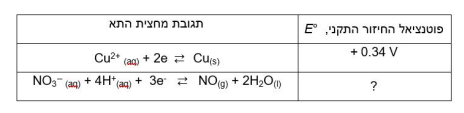
B. Calculate the value of Gibbs' standard free energy, G, for the reaction. Show calculations.
C. Calculate the equilibrium constant for reaction under standard conditions. Show calculations.
D. Calculate the cell potential if we increase only the concentration of copper ions twice At room temperature. Show calculations.
E. Nitric oxide gas, (g (NO, reacts with oxygen to form nitrogen dioxide:
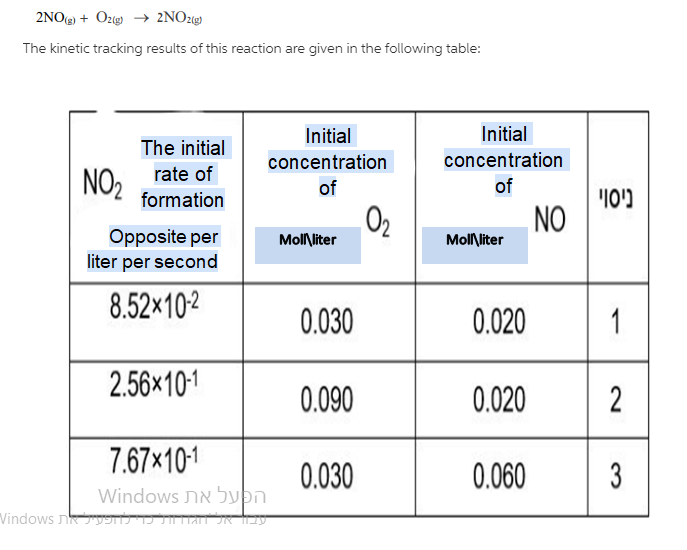
I. Determine the order of reaction towards NO and towards O2 and write the law of rhythm For response. Show calculations.
ii. Calculate the value of the rate constant for the response (including units). Show calculations.
iii .Calculate the initial rate of NO2 formation (vs. per liter per second) given The following initial concentrations: NO] = 0.04M, [O2] = 0.04M]. Show calculations.
3Cu(s) + 2NO3- (aq) + 8H+ (aq) 3Cu2+(aq) + 2NO(g) + 4H2O(1) E = +0.62 V , E +0.34 v Cu2+ (aq) + 2e e Curs) + 4H*an) + 3e - NO(g) + 2H2O(l) NO (1) ? 2NO(g) + O2(g) 2NO2(g) The kinetic tracking results of this reaction are given in the following table: The initial Initial concentration of NOrate of Initial concentration of NO Moll liter 02 Moll liter formation Opposite per liter per second 8.52x10-2 0.030 0.020 1 2.56x10-1 0.090 0.020 2 7.67x10-1 0.030 0.060 3. Windows Windows
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


