Question: thermodynamic course 13.1. The process objective can be described most simply as converting methane and water into methanol and hydrogen, and then purifying the methanol
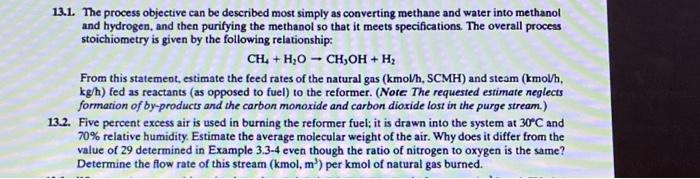
13.1. The process objective can be described most simply as converting methane and water into methanol and hydrogen, and then purifying the methanol so that it meets specifications. The overall process stoichiometry is given by the following relationship: CH4+H2OCH3OH+H2 From this statemeot, estimate the feed rates of the natural gas ( kmol/hCMH) and steam (kmollh, kg/h ) fed as reactants (as opposed to fuel) to the reformer. (Note. The requested estimate neglects formation of by-products and the carbon monoxide and carbon dioxide lost in the purge stream.) 13.2. Five percent excess air is used in burning the reformer fuel; it is drawn into the system at 30C and 70% relative humidity. Estimate the average molecular weight of the air. Why does it differ from the value of 29 determined in Example 3.3-4 even though the ratio of nitrogen to oxygen is the same? Determine the flow rate of this stream (kmol,m3) per kmol of natural gas burned. 13.1. The process objective can be described most simply as converting methane and water into methanol and hydrogen, and then purifying the methanol so that it meets specifications. The overall process stoichiometry is given by the following relationship: CH4+H2OCH3OH+H2 From this statemeot, estimate the feed rates of the natural gas ( kmol/hCMH) and steam (kmollh, kg/h ) fed as reactants (as opposed to fuel) to the reformer. (Note. The requested estimate neglects formation of by-products and the carbon monoxide and carbon dioxide lost in the purge stream.) 13.2. Five percent excess air is used in burning the reformer fuel; it is drawn into the system at 30C and 70% relative humidity. Estimate the average molecular weight of the air. Why does it differ from the value of 29 determined in Example 3.3-4 even though the ratio of nitrogen to oxygen is the same? Determine the flow rate of this stream (kmol,m3) per kmol of natural gas burned
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


