Question: Thermodynamic. I want to detailed solution. Using the density values given for the mixture below, determine the partial molar volumes of ethanol and water using
Thermodynamic. I want to detailed solution.
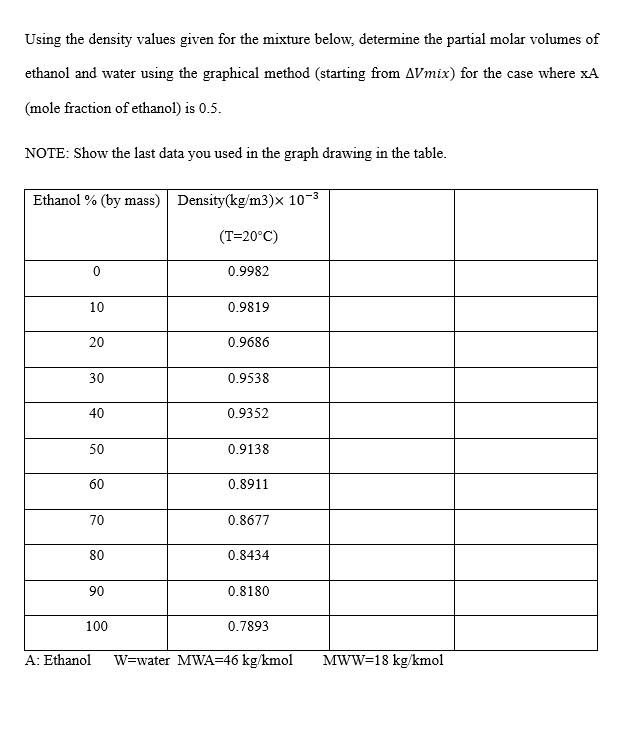
Using the density values given for the mixture below, determine the partial molar volumes of ethanol and water using the graphical method (starting from Vmix ) for the case where xA (mole fraction of ethanol) is 0.5 . NOTE: Show the last data you used in the graph drawing in the table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


