Question: Using the density values given for the mixture below, determine the partial molar volumes of ethanol and water using the graphical method (starting from AVmix)
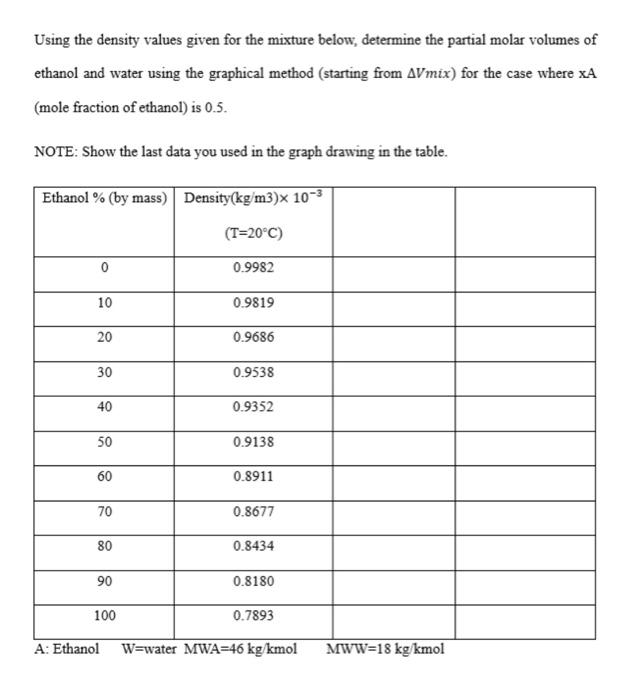
Using the density values given for the mixture below, determine the partial molar volumes of ethanol and water using the graphical method (starting from Vmix ) for the case where xA (mole fraction of ethanol) is 0.5 . NOTE: Show the last data you used in the graph drawing in the table. Using the density values given for the mixture below, determine the partial molar volumes of ethanol and water using the graphical method (starting from Vmix ) for the case where xA (mole fraction of ethanol) is 0.5 . NOTE: Show the last data you used in the graph drawing in the table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


