Question: Useful Information For thermodynamic section use the following information R, kJ/kg K Gas Cp, kJ/kg K Cs, kJ/kg K Air 0.287 1.005 0.717 Nitrogen
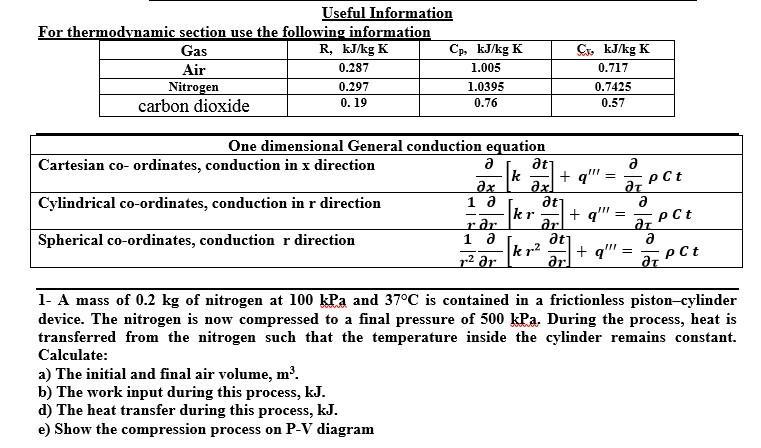
Useful Information For thermodynamic section use the following information R, kJ/kg K Gas Cp, kJ/kg K Cs, kJ/kg K Air 0.287 1.005 0.717 Nitrogen carbon dioxide 0.297 1.0395 0.7425 0. 19 0.76 0.57 One dimensional General conduction equation Cartesian co- ordinates, conduction in x direction atj k + q" axl atj kr ar at kr2 ar pCt Cylindrical co-ordinates, conduction in r direction 1 a + q" pCt Spherical co-ordinates, conduction r direction r ar 1 a + q"" a pCt p2 ar 1- A mass of 0.2 kg of nitrogen at 100 kPa and 37C is contained in a frictionless piston-cylinder device. The nitrogen is now compressed to a final pressure of 500 kPa. During the process, heat is transferred from the nitrogen such that the temperature inside the cylinder remains constant. Calculate: a) The initial and final air volume, m'. b) The work input during this process, kJ. d) The heat transfer during this process, kJ. e) Show the compression process on P-V diagram
Step by Step Solution
There are 3 Steps involved in it
To solve this problem we need to use the properties of nitrogen along with the given conditions Sinc... View full answer

Get step-by-step solutions from verified subject matter experts


