Question: Thermodynamics Complete the chart: Please show all work Data Molarity of Standardized HCl0.42M Calculations Temperature (K) 1/T(K1) Volume of HCl used (mL) Moles HCl (mol)
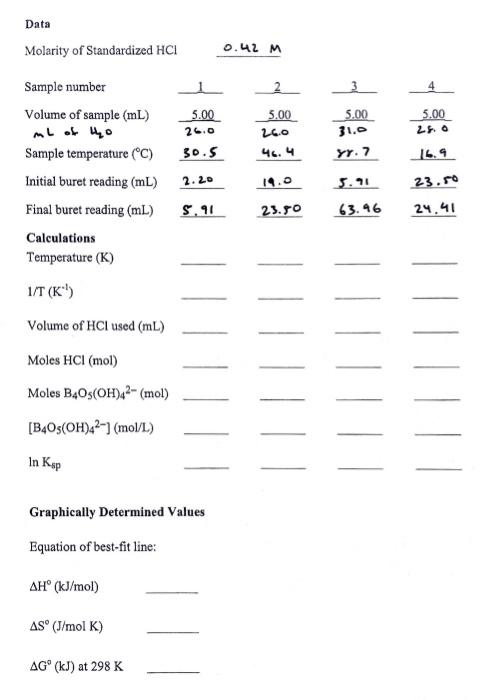
Data Molarity of Standardized HCl0.42M Calculations Temperature (K) 1/T(K1) Volume of HCl used (mL) Moles HCl (mol) Moles B4O5(OH)42(mol) [B4O5(OH)42](mol/L) lnKsp Graphically Determined Values Equation of best-fit line: H(kJ/mol)S(J/molK)G(kJ)at298K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


