Question: thermodynamics Emergency!!! Please help to do it as fast as possible! Time limitations is ONLY 1 hour. Or you just leave what you can do.
thermodynamics Emergency!!! Please help to do it as fast as possible! Time limitations is ONLY 1 hour. Or you just leave what you can do. I'll uprate your answer.
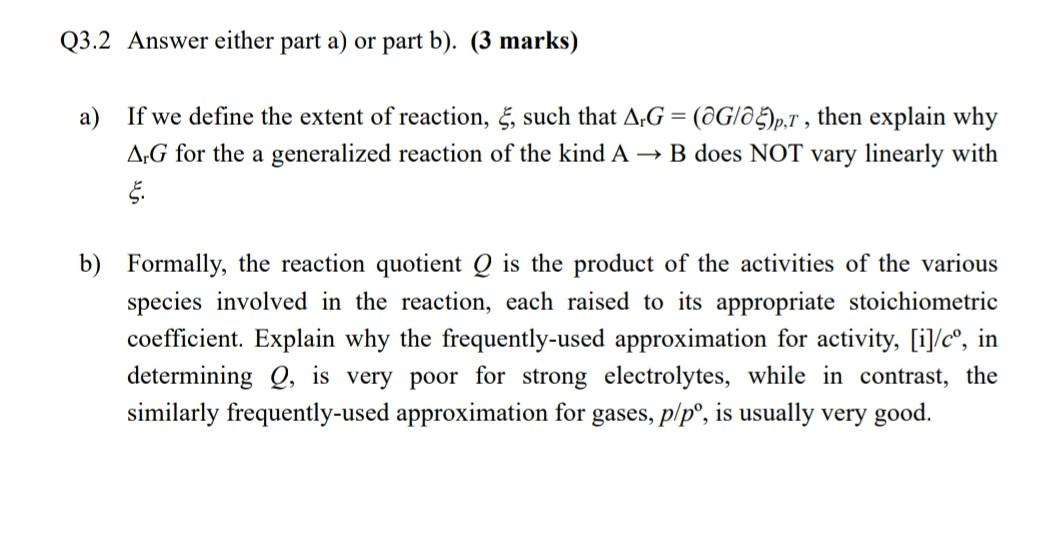
Q3.2 Answer either part a) or part b). (3 marks) = a) If we define the extent of reaction, 5, such that AG = (@G/23)p,1, then explain why ArG for the a generalized reaction of the kind A B does NOT vary linearly with . b) Formally, the reaction quotient Q is the product of the activities of the various species involved in the reaction, each raised to its appropriate stoichiometric coefficient. Explain why the frequently-used approximation for activity, [i]/c, in determining Q, is very poor for strong electrolytes, while in contrast, the similarly frequently-used approximation for gases, p/p, is usually very good
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


