Question: THERMODYNAMICS PLEASE HELP ME WITH THESE TWO PROBLEMS ILL MAKE SURE TO UPVOTE, THANK YOU! Nitrogen is contained in a rigid tank (10 m3) at
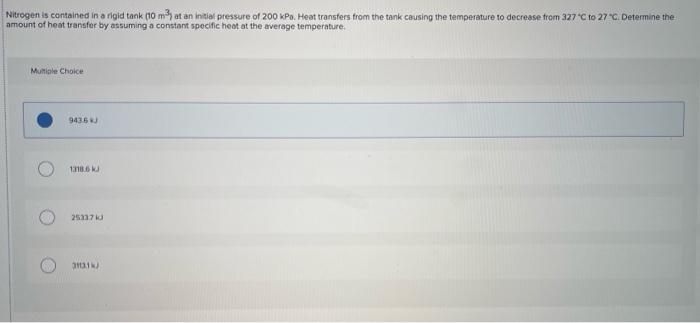
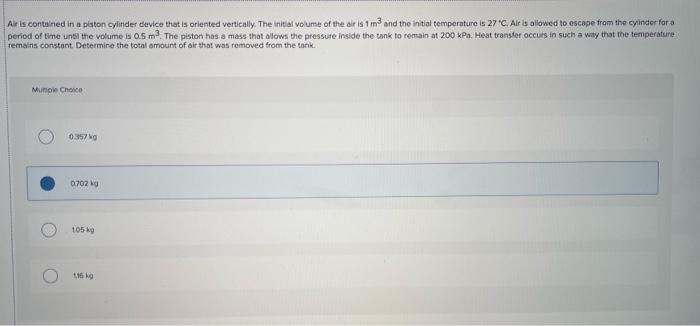
Nitrogen is contained in a rigid tank (10 m3) at an initial pressure of 200 kPa. Heat transfers from the tank causing the temperature to decrease from 327 C to 270 Determine the amount of heat transfer by assuming a constant specific heat at the average temperature, Murile Choice 9435 118.6 25317 301 Air is contained in a piston cylinder device that is oriented vertically. The initial volume of the air is 1 m3 and the initial temperature is 27 C. Air is allowed to escape from the cyinder for a period of time until the volume is 0.5 m. The piston has a mass that allows the pressure inside the tank to remain at 200 kPa. Heat transfer occurs in such a way that the temperature remains constant. Determine the total amount of air that was removed from the tank. Mungle Choice 0.357 07029 105k 116
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


