Question: Thermodynamics problem. Please explain and show all steps. Thank you 1. The following diagram and list describe a system of three interacting components. a. Write
Thermodynamics problem. Please explain and show all steps. Thank you
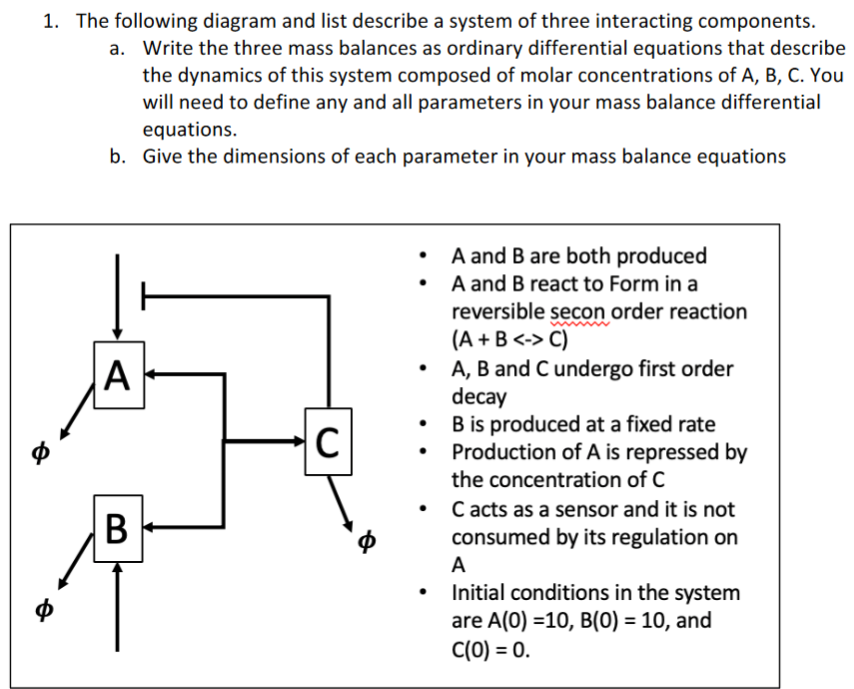
1. The following diagram and list describe a system of three interacting components. a. Write the three mass balances as ordinary differential equations that describe the dynamics of this system composed of molar concentrations of A, B, C. You will need to define any and all parameters in your mass balance differential equations. b. Give the dimensions of each parameter in your mass balance equations - A and B are both produced - A and B react to Form in a reversible secon order reaction (A+BC) - A, B and C undergo first order decay - B is produced at a fixed rate - Production of A is repressed by the concentration of C - C acts as a sensor and it is not consumed by its regulation on A - Initial conditions in the system are A(0)=10,B(0)=10, and C(0)=0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


